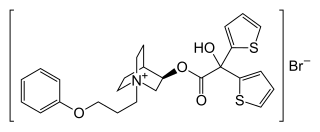
Aclidinium bromide
 | |
| Clinical data | |
|---|---|
| Trade names | Bretaris Genuair, Eklira Genuair, Tudorza Pressair |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | <5% (in system) 30% (in lung) |
| Metabolism | Ester hydrolysis |
| Elimination half-life | 2–3 hrs |
| Duration of action | >24 hrs |
| Excretion | 65% urine, 33% feces |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Aclidinium bromide (
Evidence shows that it can improve
Aclidinium is delivered via a multidose
Adverse effects
The substance is generally well tolerated. Common side effects (in more than 1% of patients) are
A small increase of
Interactions
No systematic interaction studies have been performed. It is expected that adverse effects of aclidinium increase if it is combined with other muscarinic antagonists. In clinical practice, no interactions with other COPD medications such as
Pharmacology
Mechanism of action
Aclidinium is a long-acting,
Its action at subtype M3 at the smooth muscle of the bronchioles is responsible for its desired effect: it reduces contraction of these muscles and improves the airflow.[6][7] M2 affinity is the main reason for adverse effects at the heart.[8]
Pharmacokinetics
About 30% of inhaled aclidinium are deposited in the lung.
The acid metabolite has a plasma protein binding of 87%, and the alcohol of 15%. These
Chemistry
Aclidinium is a quaternary ammonium cation with an asymmetric carbon atom. It is used as the pure R-enantiomer. The salt, aclidinium bromide, is a crystalline powder that is hardly soluble in water or ethanol.
Society and culture
Brand names
It is marketed under the brand name Tudorza Pressair in the US, Eklira Genuair in the UK, and Tudorza Genuair in Canada; licensed to Menarini under the brand name Bretaris Genuair for majority of EU member states.[9]
An inhalable combination with formoterol is marketed as Brimica Genuair[10] and Duaklir Genuair[11] in the European Union.
References
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Forest Laboratories and Almirall Announce FDA Approval of Tudorza Pressair for the Long-Term Maintenance Treatment of COPD" (Press release). Forest Laboratories. Retrieved 2012-07-24.
- S2CID 10533142.
- ^ PMID 25234126.
- hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ a b c d e Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ^ a b c FDA Professional Drug Information on Tudorza Pressair.
- ^ ISBN 978-3-7741-9846-3.
- ^ "Almirall and Menarini sign a licence agreement and commercial alliance for Aclidinium in the majority of European member states and a number of non-EU countries" (Press release). Menarini. Retrieved 2012-03-26.
- ^ "Brimica Genuair: Uses, Side Effects, Benefits/Risks". Drugs.com. 19 November 2014. Retrieved 26 July 2020.
- ^ "Duaklir Genuair: Uses, Side Effects, Benefits/Risks". Drugs.com. 19 November 2014. Retrieved 26 July 2020.
