Adenine phosphoribosyltransferase
| APRT | |||
|---|---|---|---|
Gene ontology | |||
| Molecular function | |||
| Cellular component | |||
| Biological process | |||
| Sources:Amigo / QuickGO | |||
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) | |||||||||
| Location (UCSC) | Chr 16: 88.81 – 88.81 Mb | Chr 8: 123.3 – 123.3 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
Adenine phosphoribosyltransferase (APRTase) is an
Function
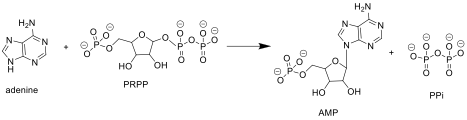
APRTase catalyzes the following reaction in the purine nucleotide salvage pathway:
Adenine + Phosphoribosyl Pyrophosphate (PRPP) → Adenylate (AMP) + Pyrophosphate (PPi)
In organisms that can synthesize
In
In
APRT is functionally related to hypoxanthine-guanine phosphoribosyltransferase (HPRT).
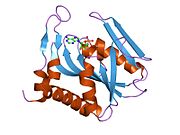
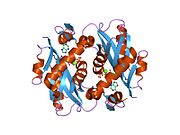
Structure
APRTase is a

- "Core" domain (residues 33-169) with five parallel β-sheets
- "Hood" domain (residues 5-34) with 2 α-helices and 2 β-sheets
- "Flexible loop" domain (residues 95-113) with 2 antiparallel β-sheets[10]

The core is highly conserved across many PRTases. The hood, which contains the
The enzyme's specificity for adenine involves hydrophobic residues
Most research on APRTase reports that Mg2+ is essential for phosphoribosyl transfer, and this is conserved across Type I PRTases.
Mechanism
APRTase proceeds via a bi bi ordered sequential mechanism, involving the formation of a ternary complex. The enzyme first binds PRPP, followed by adenine. After the phosphoribosyl transfer occurs, pyrophosphate leaves first, followed by AMP. Kinetic studies indicate that the phosphoribosyl transfer is relatively fast, while the product release (particularly the release of AMP) is rate-limiting.[9]
In human APRTase, it is thought that adenine's N9 proton is abstracted by
Deficiency
When APRTase has reduced or nonexistent activity,
ARPTase deficiency was first diagnosed in the
Type I deficiency results in a complete loss of APRTase activity and can occur in patients that are
Type II deficiency causes APRTase to have a reduced affinity for PRPP, resulting in a tenfold increase in the KM value.[6] It has been observed and studied primarily in Japan.[17]
A diagnosis of APRTase deficiency can be made by analyzing
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000198931 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000006589 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 24986359.
- ^ S2CID 40788077.
- PMID 12171924.
- ^ PMID 11535055.
- ^ PMID 11900545.
- ^ PMID 15196008.
- PMID 12171925.
- ^ PMID 12010467.
- PMID 2279281.
- ^ PMID 14767036.
- PMID 22700886.
- PMID 1353080.
- ^ PMID 20150536.
- PMID 22934314.
Further reading
- Tischfield JA, Engle SJ, Gupta PK, Bye S, Boyadjiev S, Shao C, O'Neill P, Albertini RJ, Stambrook PJ, Sahota AS (1995). "Germline and Somatic Mutation at the APRT Locus of Mice and Man". Purine and Pyrimidine Metabolism in Man VIII. Advances in Experimental Medicine and Biology. Vol. 370. pp. 661–4. PMID 7660991.
- Takeuchi H, Kaneko Y, Fujita J, Yoshida O (Apr 1993). "A case of a compound heterozygote for adenine phosphoribosyltransferase deficiency (APRT*J/APRT*Q0) leading to 2,8-dihydroxyadenine urolithiasis: review of the reported cases with 2,8-dihydroxyadenine stones in Japan". The Journal of Urology. 149 (4): 824–6. PMID 8455250.
- Ludwig H, Kuzmits R, Pietschmann H, Müller MM (Nov 1979). "Enzymes of the purine interconversion system in chronic lymphatic leukemia: decreased purine nucleoside phosphorylase and adenosine deaminase activity". Blut. 39 (5): 309–15. S2CID 6283377.
- Johnson LA, Gordon RB, Emmerson BT (Apr 1977). "Adenine phosphoribosyltransferase: a simple spectrophotometric assay and the incidence of mutation in the normal population". Biochemical Genetics. 15 (3–4): 265–72. S2CID 41264715.
- Kamatani N, Hakoda M, Otsuka S, Yoshikawa H, Kashiwazaki S (Jul 1992). "Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients". The Journal of Clinical Investigation. 90 (1): 130–5. PMID 1353080.
- Chen J, Sahota A, Laxdal T, Scrine M, Bowman S, Cui C, Stambrook PJ, Tischfield JA (Dec 1991). "Identification of a single missense mutation in the adenine phosphoribosyltransferase (APRT) gene from five Icelandic patients and a British patient". American Journal of Human Genetics. 49 (6): 1306–11. PMID 1746557.
- Mimori A, Hidaka Y, Wu VC, Tarlé SA, Kamatani N, Kelley WN, Pallela TD (Jan 1991). "A mutant allele common to the type I adenine phosphoribosyltransferase deficiency in Japanese subjects". American Journal of Human Genetics. 48 (1): 103–7. PMID 1985452.
- Chen J, Sahota A, Stambrook PJ, Tischfield JA (Jul 1991). "Polymerase chain reaction amplification and sequence analysis of human mutant adenine phosphoribosyltransferase genes: the nature and frequency of errors caused by Taq DNA polymerase". Mutation Research. 249 (1): 169–76. PMID 2067530.
- Gathof BS, Sahota A, Gresser U, Chen J, Stambrook PJ, Tischfield JA, Zöllner N (Dec 1990). "Identification of a splice mutation at the adenine phosphoribosyltransferase locus in a German family". Klinische Wochenschrift. 69 (24): 1152–5. S2CID 11791868.
- Kamatani N, Kuroshima S, Hakoda M, Palella TD, Hidaka Y (Oct 1990). "Crossovers within a short DNA sequence indicate a long evolutionary history of the APRT*J mutation" (PDF). Human Genetics. 85 (6): 600–4. S2CID 10595601.
- Kamatani N, Kuroshima S, Terai C, Hidaka Y, Palella TD, Nishioka K (Aug 1989). "Detection of an amino acid substitution in the mutant enzyme for a special type of adenine phosphoribosyltransferase (APRT) deficiency by sequence-specific protein cleavage". American Journal of Human Genetics. 45 (2): 325–31. PMID 2502918.
- Hidaka Y, Tarlé SA, Fujimori S, Kamatani N, Kelley WN, Palella TD (Mar 1988). "Human adenine phosphoribosyltransferase deficiency. Demonstration of a single mutant allele common to the Japanese". The Journal of Clinical Investigation. 81 (3): 945–50. PMID 3343350.
- Wilson JM, O'Toole TE, Argos P, Shewach DS, Daddona PE, Kelley WN (Oct 1986). "Human adenine phosphoribosyltransferase. Complete amino acid sequence of the erythrocyte enzyme". The Journal of Biological Chemistry. 261 (29): 13677–83. PMID 3531209.
- Broderick TP, Schaff DA, Bertino AM, Dush MK, Tischfield JA, Stambrook PJ (May 1987). "Comparative anatomy of the human APRT gene and enzyme: nucleotide sequence divergence and conservation of a nonrandom CpG dinucleotide arrangement". Proceedings of the National Academy of Sciences of the United States of America. 84 (10): 3349–53. PMID 3554238.
- Hidaka Y, Palella TD, O'Toole TE, Tarlé SA, Kelley WN (Nov 1987). "Human adenine phosphoribosyltransferase. Identification of allelic mutations at the nucleotide level as a cause of complete deficiency of the enzyme". The Journal of Clinical Investigation. 80 (5): 1409–15. PMID 3680503.
- Hidaka Y, Tarlé SA, O'Toole TE, Kelley WN, Palella TD (Nov 1987). "Nucleotide sequence of the human APRT gene". Nucleic Acids Research. 15 (21): 9086. PMID 3684585.
- Chen J, Sahota A, Martin GF, Hakoda M, Kamatani N, Stambrook PJ, Tischfield JA (Jun 1993). "Analysis of germline and in vivo somatic mutations in the human adenine phosphoribosyltransferase gene: mutational hot spots at the intron 4 splice donor site and at codon 87". Mutation Research. 287 (2): 217–25. PMID 7685481.
- Sahota A, Chen J, Boyadjiev SA, Gault MH, Tischfield JA (May 1994). "Missense mutation in the adenine phosphoribosyltransferase gene causing 2,8-dihydroxyadenine urolithiasis". Human Molecular Genetics. 3 (5): 817–8. PMID 7915931.
External links
- Adenine+phosphoribosyltransferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human APRT genome location and APRT gene details page in the UCSC Genome Browser.