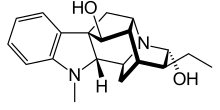
Ajmaline
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Gilurytmal, Ritmos, Aritmina |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Ajmaline (also known by trade names Gilurytmal, Ritmos, and Aritmina) is an
The compound was first isolated by
Due to the low bioavailability of ajmaline, a semisynthetic propyl derivative called prajmaline (trade name Neo-gilurythmal) was developed that induces similar effects to its predecessor but has better bioavailability and absorption.[4]
Biosynthesis
Ajmaline is widely dispersed among 25 plant genera, but is of significant concentration in the Apocynaceae family.[5] Ajmaline is a

Mechanism of action
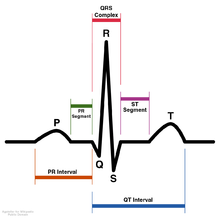
Ajmaline [10] was first discovered to lengthen the refractory period of the heart by blocking sodium ion channels,[3] but it has also been noted that it is also able to interfere with the hERG (human Ether-a-go-go-Related Gene) potassium ion channel.[11] In both cases, Ajmaline causes the action potential to become longer and ultimately leads to bradycardia. When ajmaline reversibly blocks hERG, repolarization occurs more slowly because it is harder for potassium to get out due to less unblocked channels, therefore making the RS interval longer. Ajmaline also prolongs the QR interval since it can also act as sodium channel blocker, therefore making it take longer for the membrane to depolarize in the first case. In both cases, ajmaline causes the action potential to become longer. Slower depolarization or repolarization results in a lengthened QT interval (the refractory period), and therefore makes it take more time for the membrane potential to get below the threshold level so the action potential can be re-fired. Even if another stimulus is present, action potential cannot occur again until after complete repolarization. Ajmaline causes action potentials to be prolonged, therefore slowing down firing of the conducting myocytes which ultimately slows the beating of the heart.
Diagnosis of Brugada syndrome
See also
- Hellmuth Kleinsorge (1920-2001) German medical doctor
References
- ^ Siddiqui S, Siddiqui RH (1931). "Chemical examination of the roots of Rauwolfia serpentina Benth". J. Indian Chem. Soc. 8: 667–80.
- ^ Sandilvi AN (12 April 2003). "Salimuzzaman Siddiqui: pioneer of scientific research in Pakistan". Daily Dawn. Archived from the original on 2007-09-27.
- ^ a b c Roberts MF, Wink M (1998). Alkaloids: Biochemistry, Ecology, and Medical applications. Plenum Press.
- PMID 10944783.
- PMID 26827883.
- PMID 26827882.
- S2CID 225501365.
- PMID 26827882.
- PMID 26827882.
- ^ "Ajmaleen 54 | Unani Medicine for High blood Pressure | Ajmal.pk". Dawakhana Hakim Ajmal Khan. 14 June 2019. Retrieved 2019-08-09.
- S2CID 22310415.
- PMID 19606473.
- PMID 29084732.
- PMID 12804924.
- PMID 31157372.
