Aliphatic compound
This article relies largely or entirely on a single source. (October 2022) |


In organic chemistry, hydrocarbons (compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (/ˌælɪˈfætɪk/; G. aleiphar, fat, oil). Aliphatic compounds can be saturated (in which all the C-C bonds are single requiring the structure to be completed, or 'saturated', by hydrogen) like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic.[1]
Structure
Aliphatic compounds can be
The least complex aliphatic compound is methane (CH4).
Properties
Most aliphatic compounds are
Examples of aliphatic compounds
The most important aliphatic compounds are:
- n-, iso- and cyclo-alkanes (saturated hydrocarbons)
- n-, iso- and cyclo-alkenes and -alkynes (unsaturated hydrocarbons).
Important examples of low-molecular aliphatic compounds can be found in the list below (sorted by the number of carbon-atoms):
| Formula | Name | Structural formula | Chemical classification |
|---|---|---|---|
| CH4 | Methane | 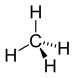 |
Alkane |
| C2H2 | Acetylene | Alkyne | |
| C2H4 | Ethylene |  |
Alkene |
| C2H6 | Ethane |  |
Alkane |
| C3H4 | Propyne | Alkyne | |
| C3H6 | Propene |
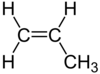 |
Alkene |
| C3H8 | Propane |  |
Alkane |
| C4H6 | 1,2-Butadiene |  |
Diene |
| C4H6 | 1-Butyne |  |
Alkyne |
| C4H8 | 1-Butene | Alkene | |
| C4H10 | Butane | 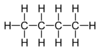 |
Alkane |
| C6H10 | Cyclohexene | Cycloalkene | |
| C5H12 | n-pentane |
Alkane | |
| C7H14 | Cycloheptane | Cycloalkane | |
| C7H14 | Methylcyclohexane | 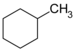 |
Cyclohexane |
| C8H8 | Cubane |  |
Prismane, Platonic hydrocarbon |
| C9H20 | Nonane | Alkane | |
| C10H12 | Dicyclopentadiene |  |
Diene, Cycloalkene |
| C10H16 | Phellandrene |   |
Terpene, Diene, Cycloalkene |
| C10H16 | α-Terpinene |
Terpene, Diene, Cycloalkene | |
| C10H16 | Limonene |  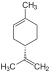 |
Terpene, Diene, Cycloalkene |
| C11H24 | Undecane | Alkane | |
| C30H50 | Squalene | Terpene, Polyene | |
| C2nH4n | Polyethylene | Alkane |
