Aluminium compounds

Reactions of aluminium
Aluminium reacts with most nonmetals upon heating, forming compounds such as
Primarily because it is corroded by dissolved
In hot concentrated
- Al + NaOH + H2O → NaAlO2 + 3/2 H2
although the aluminium species in solution is probably instead the hydrated tetrahydroxoaluminate anion, [Al(OH)4]− or [Al(H2O)2(OH)4]−.[2]
Oxidizing acids do not effectively attack high-purity aluminium because the oxide layer forms and protects the metal; aqua regia will nevertheless dissolve aluminium. This allows aluminium to be used to store reagents such as nitric acid, concentrated sulfuric acid, and some organic acids.[11]
Inorganic compounds
The vast majority of compounds, including all aluminium-containing minerals and all commercially significant aluminium compounds, feature aluminium in the oxidation state 3+. The coordination number of such compounds varies, but generally Al3+ is either six- or four-coordinate. Almost all compounds of aluminium(III) are colorless.[2]
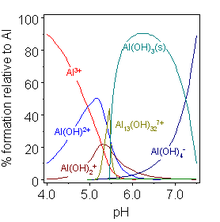
In aqueous solution, Al3+ exists as the hexaaqua cation [Al(H2O)6]3+, which has an approximate
- Al2O3 + 3 SiO2 Al2(SiO3)3
- Al2O3 + CaO Ca(AlO2)2
This behaviour of Al(OH)3 is termed amphoterism, and is characteristic of weakly basic cations that form insoluble hydroxides and whose hydrated species can also donate their protons. Further examples include Be2+, Zn2+, Ga3+, Sn2+, and Pb2+; indeed, gallium in the same group is slightly more acidic than aluminium. One effect of this is that aluminium salts with weak acids are hydrolysed in water to the aquated hydroxide and the corresponding nonmetal hydride: aluminium sulfide yields hydrogen sulfide, aluminium nitride yields ammonia, and aluminium carbide yields methane. Aluminium cyanide, acetate, and carbonate exist in aqueous solution but are unstable as such; only incomplete hydrolysis takes place for salts with strong acids, such as the halides, nitrate, and sulfate. For similar reasons, anhydrous aluminium salts cannot be made by heating their "hydrates": hydrated aluminium chloride is in fact not AlCl3·6H2O but [Al(H2O)6]Cl3, and the Al–O bonds are so strong that heating is not sufficient to break them and form Al–Cl bonds instead:[2]
- 2[Al(H2O)6]Cl3 Al2O3 + 6 HCl + 9 H2O
All four

With heavier halides, the coordination numbers are lower. The other trihalides are
- AlCl3 + 3 LiZ → 3 LiCl + AlZ3 (Z = R, NR2, N=CR2)
- AlCl3 + 4 LiZ → 3 LiCl + LiAlZ4 (Z = R, NR2, N=CR2, H)
- BF3 + AlCl3 → AlF3 + BCl3
Aluminium forms one stable oxide with the
The only stable
Rarer oxidation states
Although the great majority of aluminium compounds feature Al3+ centers, compounds with lower oxidation states are known and are sometimes of significance as precursors to the Al3+ species.
Aluminium(I)
AlF, AlCl, AlBr, and AlI exist in the gaseous phase when the respective trihalide is heated with aluminium, and at cryogenic temperatures. Their instability in the condensed phase is due to their ready disproportionation to aluminium and the respective trihalide: the reverse reaction is favored at high temperature (although even then they are still short-lived), explaining why AlF3 is more volatile when heated in the presence of aluminium, as is aluminium when heated in the presence of AlCl3.[14]
A stable derivative of aluminium monoiodide is the cyclic adduct formed with triethylamine, Al4I4(NEt3)4. Also of theoretical interest but only of fleeting existence are Al2O and Al2S. Al2O is made by heating the normal oxide, Al2O3, with silicon at 1,800 °C (3,272 °F) in a vacuum. Such materials quickly disproportionate to the starting materials.[18]
Aluminium(II)
Very simple Al(II) compounds are invoked or observed in the reactions of Al metal with oxidants. For example,
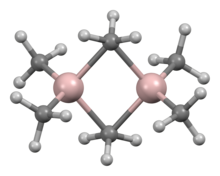
A variety of compounds of empirical formula AlR3 and AlR1.5Cl1.5 exist.
The industrially most important aluminium hydride is lithium aluminium hydride (LiAlH4), which is used in as a reducing agent in organic chemistry. It can be produced from lithium hydride and aluminium trichloride:[25]
- 4 LiH + AlCl3 → LiAlH4 + 3 LiCl
The simplest hydride, aluminium hydride or alane, is not as important. It is a polymer with the formula (AlH3)n, in contrast to the corresponding boron hydride that is a dimer with the formula (BH3)2.[25]
References
- ^ a b c Greenwood and Earnshaw, pp. 222–4
- ^ a b c d e f g h i Greenwood and Earnshaw, pp. 224–7
- ^ Greenwood and Earnshaw, pp. 112–3
- ^ King, p. 241
- ^ King, pp. 235–6
- ISBN 978-0-08-044495-6. Archivedfrom the original on 21 May 2016.
- ISBN 978-0-340-63207-9.
- ^ "Reaction of Aluminum with Water to Produce Hydrogen" (PDF). U.S. Department of Energy. 1 January 2008. Archived from the original (PDF) on 14 September 2012.
- ^ ISBN 978-0-8031-2610-7. Archivedfrom the original on 24 April 2016.
- ISBN 978-0-7503-0688-1.
- ISBN 978-3-527-30673-2.
- ISBN 978-0-89874-892-5.
- ^ a b c d e f Greenwood and Earnshaw, pp. 242–52
- ^ a b c d e f Greenwood and Earnshaw, pp. 233–7
- ISBN 978-1-136-37393-0.
- ^ Roscoe, Henry Enfield; Schorlemmer, Carl (1913). A treatise on chemistry. Macmillan. p. 718.
Aluminium forms one stable oxide, known by its mineral name corundum.
- ^ a b Greenwood and Earnshaw, pp. 252–7
- .
- S2CID 4163250.
- doi:10.1086/147348.
- ISBN 978-0-12-031151-4.
- ISBN 978-3-527-29390-2.
- ^ a b Greenwood and Earnshaw, pp. 257–67
- ^ a b Greenwood and Earnshaw, pp. 227–32
Bibliography
- ISBN 978-0-08-037941-8.
