Arylcyclohexylamine
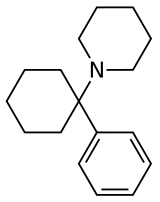
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a
History
Structure

An arylcyclohexylamine is composed of a
Pharmacology
Arylcyclohexylamines varyingly possess
These are versatile agents with a wide range of possible pharmacological activities depending on the extent and range to which chemical modifications are implemented. Thus, radically different pharmacology is possible through different structural combinations.
Notes on numbering
PCP itself is composed of three six-membered rings, which can each be substituted by a variety of groups. These are traditionally numbered in the older research as first the

However, since the widespread sale of these compounds as grey-market designer drugs, nearly all such compounds that have come to prominence either have a bare cyclohexyl ring or a 2-ketocyclohexyl ring, while the piperidine is replaced by a variety of alkyl or cycloalkyl amines and most substitution has taken place on the phenyl ring. Consequently, it is common for widely used phenyl substituted analogues such as
without the prime, even though this is technically incorrect and could lead to confusion.List of arylcyclohexylamines
| Structures | Compound | Aryl Substituent | N Group | Cyclohexyl ring | CAS number |
|---|---|---|---|---|---|
 |
PCA[24] | Phenyl | NH2 | - | 1934-71-0 |
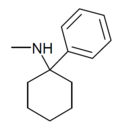 |
PCM[24] | Phenyl | Methylamino | - | 2201-16-3 |
 |
Eticyclidine | Phenyl | Ethylamino | - | 2201-15-2 |
 |
PCPr[25] | Phenyl | n-Propylamino | - | 18949-81-0 |
 |
PCiP | Phenyl | Isopropylamino | - | 1195-42-2 |
 |
PCAL [26] | Phenyl | Allylamino | - | 2185-95-7 |
 |
PCBu | Phenyl | n-Butylamino | - | 73166-29-7 |
 |
PCEOH | Phenyl | Hydroxyethylamino | - | 2201-22-1 |
 |
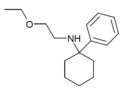
PCMEA[27] | Phenyl | Methoxyethylamino | - | 2201-57-2 |
 |
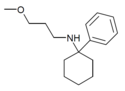
PCEEA | Phenyl | Ethoxyethylamino | - | 1072895-05-6 |
 |
PCMPA | Phenyl | Methoxypropylamino | - | 2201-58-3 |
 |
PCDM[24] | Phenyl | Dimethylamino | - | 2201-17-4 |
 |
Dieticyclidine | Phenyl | Diethylamino | - | 2201-19-6 |
 |
2-HO-PCP[6] | Phenyl | Piperidine | 2-Hydroxy | 94852-58-1 |
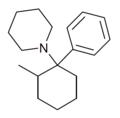 |
2-Me-PCP[28] | Phenyl | Piperidine | 2-Methyl | 59397-29-4 |
 |
2-MeO-PCP[29] | Phenyl | Piperidine | 2-Methoxy | 78636-34-7 |
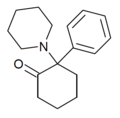 |
2-Keto-PCP | Phenyl | Piperidine | 2-Keto | 101688-16-8 |
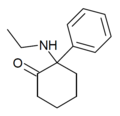 |
Eticyclidone ("O-PCE") |
Phenyl | Ethylamino | 2-Keto | 6740-82-5 |
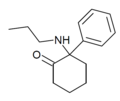 |
2-Keto-PCPr | Phenyl | n-Propylamino | 2-Keto | |
 |
4-Methyl-PCP | Phenyl | Piperidine | 4-Methyl | 19420-52-1 |
 |
4-Keto-PCP[30] | Phenyl | Piperidine | 4-Keto | 65620-13-5 |
 |
2'-Cl-PCP | o-Chlorophenyl | Piperidine | - | 2201-31-2 |
 |
3'-Cl-PCP | m-Chlorophenyl | Piperidine | - | 2201-32-3 |
 |
2'-MeO-PCP | o-Methoxyphenyl | Piperidine | - | 2201-34-5 |
 |
3'-F-PCP[31] | m-Fluorophenyl | Piperidine | - | 89156-99-0 |
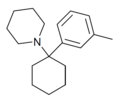 |
3'-Me-PCP[32] | m-Tolyl | Piperidine | - | 2201-30-1 |
 |
3'-Me-PCPy | m-Tolyl | Pyrrolidine | - | 1622348-63-3 |
 |
3'-NH2-PCP | m-Aminophenyl | Piperidine | - | 72242-00-3 |
 |
3'-HO-PCP | m-Hydroxyphenyl | Piperidine | - | 79787-43-2 |
 |
3'-MeO-PCP | m-Methoxyphenyl | Piperidine | - | 72242-03-6 |
 |
3',4'-MD-PCP | 3,4-Methylenedioxyphenyl | Piperidine | - | |
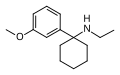 |
3'-MeO-PCE | m-Methoxyphenyl | Ethylamino | - | 1364933-80-1 |
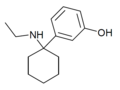 |
3'-HO-PCE | m-Hydroxyphenyl | Ethylamino | - | |
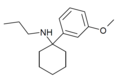 |
3'-MeO-PCPr | m-Methoxyphenyl | n-Propylamino | - | 1364933-81-2 |
 |
3'-HO-PCPr | m-Hydroxyphenyl | n-Propylamino | - | |
 |
3',4'-MD-PCPr | 3,4-Methylenedioxyphenyl | n-Propylamino | - | |
 |
3'-MeO-PCPy[32] | m-Methoxyphenyl | Pyrrolidine | - | 1364933-79-8 |
 |
4'-HO-PCP | p-Hydroxyphenyl | Piperidine | - | 66568-88-5 |
 |
Methoxydine (4'-MeO-PCP) |
p-Methoxyphenyl | Piperidine | - | 2201-35-6 |
 |
4'-MeO-PCE | p-Methoxyphenyl | Ethylamino | - | |
 |
4'-F-PCP[31] | p-Fluorophenyl | Piperidine | - | 22904-99-0 |
 |
4'-F-PCPy | p-Fluorophenyl | Pyrrolidine | - | |
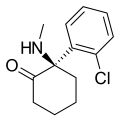 |
Arketamine | o-Chlorophenyl | Methylamino | 2-Keto | 33643-49-1 |
 |
Deschloroketamine | Phenyl | Methylamino | 2-Keto | 7063-30-1 |
 |
Esketamine | o-Chlorophenyl | Methylamino | 2-Keto | 33643-46-8 |
 |
Ketamine | o-Chlorophenyl | Methylamino | 2-Keto | 6740-88-1 |
 |
Hydroxynorketamine | o-Chlorophenyl | NH2 | 2-Keto, 6-Hydroxy | 81395-70-2 |
 |
Ethketamine | o-Chlorophenyl | Ethylamino | 2-Keto | 1354634-10-8 |
 |
NPNK | o-Chlorophenyl | n-Propylamino | 2-Keto | 2749326-65-4 |
 |
Methoxyketamine | o-Methoxyphenyl | Methylamino | 2-Keto | 7063-51-6 |
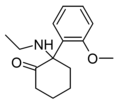 |
2-MeO-NEK[33] | o-Methoxyphenyl | Ethylamino | 2-Keto | |
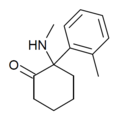 |
oMDCK[34] | o-Tolyl | Methylamino | 2-Keto | 7063-37-8 |
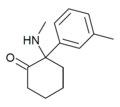 |
mMDCK | m-Tolyl | Methylamino | 2-Keto | |
 |
meta-Ketamine | m-Chlorophenyl | Methylamino | 2-Keto | 7063-53-8 |
 |
iso-Ketamine | o-Chlorophenyl | Methylamino | 4-Keto | |
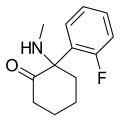 |
2-Fluorodeschloroketamine | o-Fluorophenyl | Methylamino | 2-Keto | 111982-50-4 |
 |
3-Fluorodeschloroketamine | m-Fluorophenyl | Methylamino | 2-Keto | 2657761-23-2 |
 |
Bromoketamine |
o-Bromophenyl | Methylamino | 2-Keto | 120807-70-7 |
 |
TFMDCK | o-Trifluoromethylphenyl | Methylamino | 2-Keto | 1782149-73-8 |
 |
SN 35210[35] | o-Chlorophenyl | Carbomethoxybutylamino | 2-Keto | 1450615-41-4 |
 |
Methoxetamine | m-Methoxyphenyl | Ethylamino | 2-Keto | 1239943-76-0 |
 |
Methoxmetamine | m-Methoxyphenyl | Methylamino | 2-Keto | 1781829-56-8 |
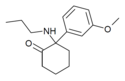 |
Methoxpropamine | m-Methoxyphenyl | n-Propylamino | 2-Keto | 2504100-71-2 |
 |
MXiPr |
m-Methoxyphenyl | i-Propylamino | 2-Keto | |
 |
Ethoxetamine | m-Ethoxyphenyl | Ethylamino | 2-Keto | |
 |
Deoxymethoxetamine (3-Me-2'-Oxo-PCE) | m-Tolyl | Ethylamino | 2-Keto | 2666932-45-0 |
 |
Br-MXE | 2-bromo-5-methoxyphenyl | Ethylamino | 2-Keto | |
 |
Hydroxetamine (HXE) | m-Hydroxyphenyl | Ethylamino | 2-Keto | 1620054-73-0 |
 |
HXM | m-Hydroxyphenyl | Methylamino | 2-Keto | |
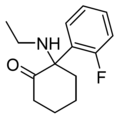 |
2F-NENDCK | o-Fluorophenyl | Ethylamino | 2-Keto | |
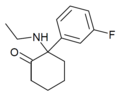 |
Fluorexetamine (FXE) | m-Fluorophenyl | Ethylamino | 2-Keto | |
 |
Phencyclidine (PCP) | Phenyl | Piperidine | - | 77-10-1 |
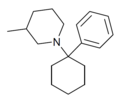 |
PC3MP | Phenyl | 3-Methylpiperidine | - | 2201-41-4 |
 |
PC4MP | Phenyl | 4-Methylpiperidine | - | 2201-42-5 |
 |
Rolicyclidine (PCPy) | Phenyl | Pyrrolidine | - | 2201-39-0 |
 |
PCDMPy | Phenyl | 3,3-Dimethylpyrrolidine | - | |
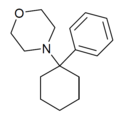 |
PCMo | Phenyl | Morpholine | - | 2201-40-3 |
 |
Methoxy-PCM[7] (2'-MeO-PCMo) | o-Methoxyphenyl | Morpholine | - | 1314323-88-0 |
 |
3'-MeO-PCMo | m-Methoxyphenyl | Morpholine | - | 138873-80-0 |
 |
4'-MeO-PCMo | p-Methoxyphenyl | Morpholine | - | |
 |
Methyl-PCM[36] (4'-Me-PCMo) | p-Tolyl | Morpholine | - | 120803-52-3 |
 |
Hydroxy-methyl-PCM | 2-Methyl-4-hydroxyphenyl | Morpholine | - | 1314323-89-1 |
 |
PYCP [37] | 2-Pyridinyl | Piperidine | - | |
 |
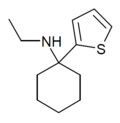
TCM | 2-Thienyl | Methylamino | - | 139401-07-3 |
 |
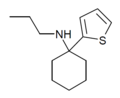
TCE | 2-Thienyl | Ethylamino | - | 101589-62-2 |
 |
TCPr [38] | 2-Thienyl | Propylamino | - | |
 |
Tenocyclidine (TCP) | 2-Thienyl | Piperidine | - | 21500-98-1 |
 |
T3CP | 3-Thienyl | Piperidine | - | 19420-50-9 |
 |
TCPy | 2-Thienyl | Pyrrolidine | - | 22912-13-6 |
 |
Tiletamine | 2-Thienyl | Ethylamino | 2-Keto | 14176-49-9 |
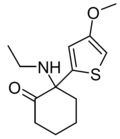 |
MXTE | 4-Methoxy-2-thienyl | Ethylamino | 2-Keto | |
 |
Gacyclidine | 2-Thienyl | Piperidine | 2-Methyl | 68134-81-6 |
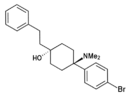 |
BDPC |
p-Bromophenyl | Dimethylamino | 4-Phenethyl-4-hydroxy | 77239-98-6 |
 |
C-8813 | p-Bromophenyl | Dimethylamino | 4-(thiophen-2-yl)ethyl-4-hydroxy | 616898-54-5 |
 |
Dimetamine[39] | p-Tolyl | Dimethylamino | 4-Keto | 65619-06-9 |
 |
3''-OH-2'-Me-PCP [40] | o-Tolyl | 3-Hydroxypiperidine | - | |
 |
4''-Ph-4''-OH-PCP [41] | Phenyl | 4-Phenyl-4-hydroxypiperidine | - | 77179-39-6 |
 |
BTCP[42] | Benzothiophen-2-yl | Piperidine | - | 112726-66-6 |
 |
BTCPy[10] | Benzothiophen-2-yl | Pyrrolidine | - | |
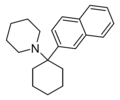 |
GK-189[43] | Naphthalen-2-yl | Piperidine | - | 81490-58-6 |
Related compounds
Other similar compounds exist where the base ring has been varied, or the amine chain replaced with other groups.[44] More cycloalkane ring sizes have been experimented with than just purely thinking in terms of the cyclohexylamine. The cyclopentyl homologue of PCP is active with around one-tenth the potency,[45] while the cycloheptyl and cyclooctyl derivatives are inactive, though some substituted arylcycloheptylamines retain activity.[46] The requisite cycloalkylketone is reacted with PhMgBr; 3° alcohol is then reacted with NaN3; azide then reduced with LAH. Then in the final step the piperidine ring is constructed with 1-5-dibromo-pentane.[47] Other compounds are known where the cyclohexyl base ring is replaced by rings such as norbornyl, adamantyl,[48] tetralin, oxane, thiane [49] or piperidine.[50] Conformationally constrained analogs have been prepared and researched by Morieti et al.[51]
| Structure | Compound | Aryl Substituent | N Group | Base ring | CAS number |
|---|---|---|---|---|---|
 |
PCPEP | Phenyl | Piperidine | Cyclopentyl | 23036-19-3 |
 |
3F-PCHEPy | 3-Fluorophenyl | Pyrrolidine | Cycloheptyl | |
 |
3-MeO-PBCHP | 3-Methoxyphenyl | Piperidine | Bicyclo[2.2.1]heptane | |
 |
PADP (P2AP) | Phenyl | Piperidine | Adamantyl | 72241-99-7 |
 |
3-MeO-PTP | 3-Methoxyphenyl | Piperidine | Tetralin | |
 |
HHFA | Fused phenyl | Amino | Hexahydrofluorene | |
 |
DHPQ | Phenyl | Decahydroquinoline | ||
 |
POXP | Phenyl | Piperidine | Oxane | |
 |
PTHP | Phenyl | Piperidine | Thiane | |
 |
MPBPip | Phenyl | Piperidine | N-Methylpiperidine | 36882-04-9 |
 |
BnCP | Benzyl | Piperidine | Cyclohexyl | 22912-07-8 |
 |
YNCP | Ethynyl | Piperidine | Cyclohexyl | 51165-02-7 |
 |
ALCP | Allyl | Piperidine | Cyclohexyl | 7418-80-6 |
 |
Piritramide | Replaced by carboxamide | Piperidine | N-(3-cyano-3,3-diphenylpropyl)piperidine | 302-41-0 |
 |
PRE-084 | Phenyl | Morpholinylethylcarboxylate | Cyclohexyl | 138847-85-5 |
 |
Clofenciclan | p-Chlorophenyl | Diethylaminoethoxy | Cyclohexyl | 5632-52-0 |
References
- ^ "4-(1-phenyl-cyclohexyl)-morpholine". CAS Number Search - chemsrc.com. chemsrc. Retrieved 15 March 2021.
- ^ PMID 24678061.
- ISBN 0-444-20525-X.
- PMID 30105474.
- PMID 30196446.
- ^ S2CID 29006399.
- ^ S2CID 24650035.
- ^ S2CID 7672918.
- PMID 6086884.
- ^ PMID 8098066.
- S2CID 22266452.
- ^ PMID 15852061.
- PMID 8737636.
- S2CID 42842315.
- S2CID 5402425.
- PMID 24251803.
- S2CID 24287727.
- PMID 24381602.
- S2CID 23966936.
- PMID 23214193.
- PMID 27121715.
- PMID 513062.
- S2CID 54306053.
- ^ PMID 2329567.
- PMID 18035363.
- PMID 6481761.
- PMID 19022226.
- PMID 1875352.
- S2CID 10370245.
- S2CID 226234538.
- ^ .
- ^ PMID 23554350.
- S2CID 83463722.
- ^ WO 2021134086, Kruegel AC, Sames D, Hashimoto K, "Arylcyclohexylamine derivatives and their use in the treatment of psychiatric disorders", published 1 July 2021, assigned to Gilgamesh Pharmaceuticals, Inc. and The Trustees Of Columbia University In The City Of New York.
- S2CID 36017002.
- S2CID 8094521.
- PMID 21334205.
- S2CID 1599386.
- PMID 7381841.
- S2CID 24803623.
- PMID 7241506.
- PMID 3384005.
- ^ Kamenka JM, et al. Substituted cyclic amines and pharmaceutical composition containing them. Patent US5248686, 28 September 1993
- ^ Wallach JV. Structure activity relationship (SAR) studies of arylcycloalkylamines as N-methyl-D-aspartate receptor antagonists. PhD. Thesis, University of the Sciences in Philadelphia, 19 Dec 2014.
- PMID 975751.
- PMID 24773376.
- PMID 7310819.
- PMID 6834381.
- ^ Sisco E, Urbas A. Identification and Characterization of Designer Phencyclidines (PCPs) in Forensic Casework
- ^ Gerhard O, Eberhard E. 4-amino-piperidines. US3311624A
- PMID 9484497.
Further reading
- Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. PMID 24678061.
