BRCA1
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) |
| ||||||||
| RefSeq (protein) | |||||||||
| Location (UCSC) | Chr 17: 43.04 – 43.17 Mb | Chr 11: 101.38 – 101.44 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
Breast cancer type 1 susceptibility protein is a
BRCA1 and BRCA2 are unrelated proteins,[10] but both are normally expressed in the cells of breast and other tissue, where they help repair damaged DNA, or destroy cells if DNA cannot be repaired. They are involved in the repair of chromosomal damage with an important role in the error-free repair of DNA double-strand breaks.[11][12] If BRCA1 or BRCA2 itself is damaged by a BRCA mutation, damaged DNA is not repaired properly, and this increases the risk for breast cancer.[13][14] BRCA1 and BRCA2 have been described as "breast cancer susceptibility genes" and "breast cancer susceptibility proteins". The predominant allele has a normal, tumor suppressive function whereas high penetrance mutations in these genes cause a loss of tumor suppressive function which correlates with an increased risk of breast cancer.[15]
BRCA1 combines with other tumor suppressors, DNA damage sensors and signal transducers to form a large multi-subunit protein complex known as the BRCA1-associated genome surveillance complex (BASC).[16] The BRCA1 protein associates with RNA polymerase II, and through the C-terminal domain, also interacts with histone deacetylase complexes. Thus, this protein plays a role in transcription, and DNA repair of double-strand DNA breaks[14] ubiquitination, transcriptional regulation as well as other functions.[17]
Methods to test for the likelihood of a patient with mutations in BRCA1 and BRCA2 developing cancer were covered by
Discovery
The first evidence for the existence of a gene encoding a DNA repair enzyme involved in breast cancer susceptibility was provided by Mary-Claire King's laboratory at UC Berkeley in 1990.[22] Four years later, after an international race to find it,[23] the gene was cloned in 1994 by scientists at University of Utah, National Institute of Environmental Health Sciences (NIEHS) and Myriad Genetics.[18][24]
Gene location
The human BRCA1 gene is located on the long (q) arm of
Protein structure
The BRCA1 protein contains the following domains:[26]
- Zinc finger, C3HC4 type (RING finger)
- BRCA1 C Terminus (BRCT) domain
This protein also contains
The human
BRCA1 is unrelated to

Zinc ring finger domain
The
The ring domain is an important element of ubiquitin E3 ligases, which catalyze protein ubiquitination. Ubiquitin is a small regulatory protein found in all tissues that direct proteins to compartments within the cell. BRCA1 polypeptides, in particular, Lys-48-linked polyubiquitin chains are dispersed throughout the resting cell nucleus, but at the start of DNA replication, they gather in restrained groups that also contain BRCA2 and BARD1. BARD1 is thought to be involved in the recognition and binding of protein targets for ubiquitination.[31] It attaches to proteins and labels them for destruction. Ubiquitination occurs via the BRCA1 fusion protein and is abolished by zinc chelation.[30] The enzyme activity of the fusion protein is dependent on the proper folding of the ring domain.[citation needed]
Serine cluster domain
BRCA1 serine cluster domain (SCD) spans amino acids 1280–1524. A portion of the domain is located in exons 11–13. High rates of mutation occur in exons 11–13. Reported phosphorylation sites of BRCA1 are concentrated in the SCD, where they are phosphorylated by
BRCT domains
The dual repeat
Function and mechanism
BRCA1 is part of a complex that repairs
In the nucleus of many types of normal cells, the BRCA1 protein interacts with RAD51 during repair of DNA double-strand breaks.[38] These breaks can be caused by natural radiation or other exposures, but also occur when chromosomes exchange genetic material (homologous recombination, e.g., "crossing over" during meiosis). The BRCA2 protein, which has a function similar to that of BRCA1, also interacts with the RAD51 protein. By influencing DNA damage repair, these three proteins play a role in maintaining the stability of the human genome.[39]
BRCA1 is also involved in another type of DNA repair, termed mismatch repair. BRCA1 interacts with the DNA mismatch repair protein MSH2.[40] MSH2, MSH6, PARP and some other proteins involved in single-strand repair are reported to be elevated in BRCA1-deficient mammary tumors.[41]
A protein called valosin-containing protein (VCP, also known as p97) plays a role to recruit BRCA1 to the damaged DNA sites. After ionizing radiation, VCP is recruited to DNA lesions and cooperates with the ubiquitin ligase RNF8 to orchestrate assembly of signaling complexes for efficient DSB repair.[42] BRCA1 interacts with VCP.[43] BRCA1 also interacts with c-Myc, and other proteins that are critical to maintain genome stability.[44]
BRCA1 directly binds to DNA, with higher affinity for branched DNA structures. This ability to bind to DNA contributes to its ability to inhibit the nuclease activity of the
Formaldehyde and acetaldehyde are common environmental sources of DNA cross links that often require repairs mediated by BRCA1 containing pathways.[48]
This DNA repair function is essential; mice with loss-of-function mutations in both BRCA1 alleles are not viable, and as of 2015 only two adults were known to have loss-of-function mutations in both alleles (leading to FA-S); both had congenital or developmental issues, and both had cancer. One was presumed to have survived to adulthood because one of the BRCA1 mutations was
Transcription
BRCA1 was shown to co-purify with the human RNA Polymerase II holoenzyme in HeLa extracts, implying it is a component of the holoenzyme.[50] Later research, however, contradicted this assumption, instead showing that the predominant complex including BRCA1 in HeLa cells is a 2 megadalton complex containing SWI/SNF.[51] SWI/SNF is a chromatin remodeling complex. Artificial tethering of BRCA1 to chromatin was shown to decondense heterochromatin, though the SWI/SNF interacting domain was not necessary for this role.[47] BRCA1 interacts with the NELF-B (COBRA1) subunit of the NELF complex.[47]
Mutations and cancer risk
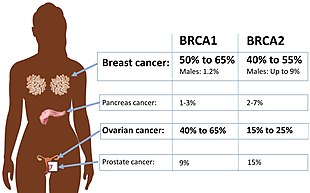
Certain variations of the BRCA1 gene lead to an increased risk for breast cancer as part of a hereditary breast–ovarian cancer syndrome. Researchers have identified hundreds of mutations in the BRCA1 gene, many of which are associated with an increased risk of cancer. Females with an abnormal BRCA1 or BRCA2 gene have up to an 80% risk of developing breast cancer by age 90; increased risk of developing ovarian cancer is about 55% for females with BRCA1 mutations and about 25% for females with BRCA2 mutations.[53]
These mutations can be changes in one or a small number of DNA base pairs (the building-blocks of DNA), and can be identified with PCR and DNA sequencing.[54]
In some cases, large segments of DNA are rearranged. Those large segments, also called large rearrangements, can be a deletion or a duplication of one or several exons in the gene. Classical methods for mutation detection (sequencing) are unable to reveal these types of mutation.
Some results suggest that
A mutated BRCA1 gene usually makes a protein that does not function properly. Researchers believe that the defective BRCA1 protein is unable to help fix DNA damage leading to mutations in other genes. These mutations can accumulate and may allow cells to grow and divide uncontrollably to form a tumor. Thus, BRCA1 inactivating mutations lead to a predisposition for cancer.[citation needed]
BRCA1 mRNA
In addition to breast cancer, mutations in the BRCA1 gene also increase the risk of ovarian and prostate cancers. Moreover, precancerous lesions (dysplasia) within the fallopian tube have been linked to BRCA1 gene mutations. Pathogenic mutations anywhere in a model pathway containing BRCA1 and BRCA2 greatly increase risks for a subset of leukemias and lymphomas.[14]
Women who have inherited a defective BRCA1 or BRCA2 gene are at a greatly elevated risk to develop breast and ovarian cancer. Their risk of developing breast and/or ovarian cancer is so high, and so specific to those cancers, that many mutation carriers choose to have prophylactic surgery. There has been much conjecture to explain such apparently striking tissue specificity. Major determinants of where BRCA1/2 hereditary cancers occur are related to tissue specificity of the cancer pathogen, the agent that causes chronic inflammation or the carcinogen. The target tissue may have receptors for the pathogen, may become selectively exposed to an inflammatory process or to a carcinogen. An innate genomic deficit in a tumor suppressor gene impairs normal responses and exacerbates the susceptibility to disease in organ targets. This theory also fits data for several tumor suppressors beyond BRCA1 or BRCA2. A major advantage of this model is that it suggests there may be some options in addition to prophylactic surgery.[62]
As aforementioned, biallelic and homozygous inheritance of the BRCA1 gene leads to FA-S, which is almost always an embryonically lethal condition.
Low expression of BRCA1 in breast and ovarian cancers
BRCA1 expression is reduced or undetectable in the majority of high grade, ductal breast cancers.[63] It has long been noted that loss of BRCA1 activity, either by germ-line mutations or by down-regulation of gene expression, leads to tumor formation in specific target tissues. In particular, decreased BRCA1 expression contributes to both sporadic and inherited breast tumor progression.[64] Reduced expression of BRCA1 is tumorigenic because it plays an important role in the repair of DNA damages, especially double-strand breaks, by the potentially error-free pathway of homologous recombination.[65] Since cells that lack the BRCA1 protein tend to repair DNA damages by alternative more error-prone mechanisms, the reduction or silencing of this protein generates mutations and gross chromosomal rearrangements that can lead to progression to breast cancer.[65]
Similarly, BRCA1 expression is low in the majority (55%) of sporadic epithelial ovarian cancers (EOCs) where EOCs are the most common type of ovarian cancer, representing approximately 90% of ovarian cancers.[66] In serous ovarian carcinomas, a sub-category constituting about 2/3 of EOCs, low BRCA1 expression occurs in more than 50% of cases.[67] Bowtell[68] reviewed the literature indicating that deficient homologous recombination repair caused by BRCA1 deficiency is tumorigenic. In particular this deficiency initiates a cascade of molecular events that sculpt the evolution of high-grade serous ovarian cancer and dictate its response to therapy. Especially noted was that BRCA1 deficiency could be the cause of tumorigenesis whether due to BRCA1 mutation or any other event that causes a deficiency of BRCA1 expression.
In addition to its role in repairing DNA damages, BRCA1 facilitates apoptosis in breast and ovarian cell lines when cells are stressed by agents, including ionizing radiation, that cause DNA damages.[69] Repair of DNA damages and apoptosis are two enzymatic processes essential for maintaining genome integrity in humans. Cells that are deficient in DNA repair tend to accumulate DNA damages, and when such cells are also defective in apoptosis they tend to survive even with excess DNA damage.[70] Replication of DNA in such cells leads to mutations and these mutations may cause cancer. Thus BRCA1 appears to have two roles related to the prevention of cancer, where one role is to promote repair of a specific class of damages and the second role is to induce apoptosis if the level of such DNA damage is beyond the cell’s repair capability[70]
Mutation of BRCA1 in breast and ovarian cancer
Only about 3%–8% of all women with breast cancer carry a mutation in BRCA1 or BRCA2.[71] Similarly, BRCA1 mutations are only seen in about 18% of ovarian cancers (13% germline mutations and 5% somatic mutations).[72]
Thus, while BRCA1 expression is low in the majority of these cancers, BRCA1 mutation is not a major cause of reduced expression. Certain latent viruses, which are frequently detected in breast cancer tumors, can decrease the expression of the BRCA1 gene and cause the development of breast tumors.[73]
BRCA1 promoter hypermethylation in breast and ovarian cancer
BRCA1 promoter hypermethylation was present in only 13% of unselected primary breast carcinomas.[74] Similarly, BRCA1 promoter hypermethylation was present in only 5% to 15% of EOC cases.[66]
Thus, while BRCA1 expression is low in these cancers, BRCA1 promoter methylation is only a minor cause of reduced expression.
MicroRNA repression of BRCA1 in breast cancers
There are a number of specific microRNAs, when overexpressed, that directly reduce expression of specific DNA repair proteins (see MicroRNA section DNA repair and cancer) In the case of breast cancer, microRNA-182 (miR-182) specifically targets BRCA1.[75] Breast cancers can be classified based on receptor status or histology, with triple-negative breast cancer (15%–25% of breast cancers), HER2+ (15%–30% of breast cancers), ER+/PR+ (about 70% of breast cancers), and Invasive lobular carcinoma (about 5%–10% of invasive breast cancer). All four types of breast cancer were found to have an average of about 100-fold increase in miR-182, compared to normal breast tissue.[76] In breast cancer cell lines, there is an inverse correlation of BRCA1 protein levels with miR-182 expression.[75] Thus it appears that much of the reduction or absence of BRCA1 in high grade ductal breast cancers may be due to over-expressed miR-182.
In addition to miR-182, a pair of almost identical microRNAs, miR-146a and miR-146b-5p, also repress BRCA1 expression. These two microRNAs are over-expressed in triple-negative tumors and their over-expression results in BRCA1 inactivation.[77] Thus, miR-146a and/or miR-146b-5p may also contribute to reduced expression of BRCA1 in these triple-negative breast cancers.
MicroRNA repression of BRCA1 in ovarian cancers
In both serous tubal intraepithelial carcinoma (the precursor lesion to high grade serous ovarian carcinoma (HG-SOC)), and in HG-SOC itself, miR-182 is overexpressed in about 70% of cases.[78] In cells with over-expressed miR-182, BRCA1 remained low, even after exposure to ionizing radiation (which normally raises BRCA1 expression).[78] Thus much of the reduced or absent BRCA1 in HG-SOC may be due to over-expressed miR-182.
Another microRNA known to reduce expression of BRCA1 in ovarian cancer cells is miR-9.[66] Among 58 tumors from patients with stage IIIC or stage IV serous ovarian cancers (HG-SOG), an inverse correlation was found between expressions of miR-9 and BRCA1,[66] so that increased miR-9 may also contribute to reduced expression of BRCA1 in these ovarian cancers.
Deficiency of BRCA1 expression is likely tumorigenic
DNA damage appears to be the primary underlying cause of cancer,
Germ-line mutations and founder effect
All germ-line BRCA1 mutations identified to date have been inherited, suggesting the possibility of a large "founder" effect in which a certain mutation is common to a well-defined population group and can, in theory, be traced back to a common ancestor. Given the complexity of mutation screening for BRCA1, these common mutations may simplify the methods required for mutation screening in certain populations. Analysis of mutations that occur with high frequency also permits the study of their clinical expression.[83] Examples of manifestations of a founder effect are seen among Ashkenazi Jews. Three mutations in BRCA1 have been reported to account for the majority of Ashkenazi Jewish patients with inherited BRCA1-related breast and/or ovarian cancer: 185delAG, 188del11 and 5382insC in the BRCA1 gene.[84][85] In fact, it has been shown that if a Jewish woman does not carry a BRCA1 185delAG, BRCA1 5382insC founder mutation, it is highly unlikely that a different BRCA1 mutation will be found.[86] Additional examples of founder mutations in BRCA1 are given in Table 1 (mainly derived from[83]).
| Population or subgroup | BRCA1 mutation(s)[87] | Reference(s) |
|---|---|---|
| African-Americans | 943ins10, M1775R | [88] |
| Afrikaners | E881X, 1374delC | [89][90] |
| Ashkenazi Jewish | 185delAG, 188del11, 5382insC | [84][85] |
| Austrians | 2795delA, C61G, 5382insC, Q1806stop | [91] |
| Belgians | 2804delAA, IVS5+3A>G | [92][93] |
| Dutch | Exon 2 deletion, exon 13 deletion, 2804delAA | [92][94][95] |
| Finns | 3745delT, IVS11-2A>G | [96][97] |
| French | 3600del11, G1710X | [98] |
| French Canadians | C4446T | [99] |
| Germans | 5382insC, 4184del4 | [100][101] |
| Greeks | 5382insC | [102] |
| Hungarians | 300T>G, 5382insC, 185delAG | [103] |
| Italians | 5083del19 | [104] |
| Japanese | L63X, Q934X | [105] |
| Native North Americans | 1510insG, 1506A>G | [106] |
| Northern Irish | 2800delAA | [107] |
| Norwegians | 816delGT, 1135insA, 1675delA, 3347delAG | [108][109] |
| Pakistanis | 2080insA, 3889delAG, 4184del4, 4284delAG, IVS14-1A>G | [110] |
| Poles | 300T>G, 5382insC, C61G, 4153delA | [111][112] |
| Russians | 5382insC, 4153delA | [113] |
| Scots | 2800delAA | [107][114] |
| Spaniards | R71G | [115][116] |
| Swedes | Q563X, 3171ins5, 1201del11, 2594delC | [88][117] |
Female fertility
As women age, reproductive performance declines, leading to menopause. This decline is tied to a reduction in the number of ovarian follicles. Although about 1 million oocytes are present at birth in the human ovary, only about 500 (about 0.05%) of these ovulate. The decline in ovarian reserve appears to occur at a constantly increasing rate with age,[118] and leads to nearly complete exhaustion of the reserve by about age 52. As ovarian reserve and fertility decline with age, there is also a parallel increase in pregnancy failure and meiotic errors, resulting in chromosomally abnormal conceptions.[119]
Women with a germ-line BRCA1 mutation appear to have a diminished oocyte reserve and decreased fertility compared to normally aging women.[120] Furthermore, women with an inherited BRCA1 mutation undergo menopause prematurely.[121] Since BRCA1 is a key DNA repair protein, these findings suggest that naturally occurring DNA damages in oocytes are repaired less efficiently in women with a BRCA1 defect, and that this repair inefficiency leads to early reproductive failure.[120]
As noted above, the BRCA1 protein plays a key role in homologous recombinational repair. This is the only known cellular process that can accurately repair DNA double-strand breaks. DNA double-strand breaks accumulate with age in humans and mice in primordial follicles.[122] Primordial follicles contain oocytes that are at an intermediate (prophase I) stage of meiosis. Meiosis is the general process in eukaryotic organisms by which germ cells are formed, and it is likely an adaptation for removing DNA damages, especially double-strand breaks, from germ line DNA.[123] (Also see article Meiosis). Homologous recombinational repair employing BRCA1 is especially promoted during meiosis. It was found that expression of four key genes necessary for homologous recombinational repair of DNA double-strand breaks (BRCA1, MRE11, RAD51 and ATM) decline with age in the oocytes of humans and mice,[122] leading to the hypothesis that DNA double-strand break repair is necessary for the maintenance of oocyte reserve and that a decline in efficiency of repair with age plays a role in ovarian aging.
Cancer chemotherapy
Non-small cell lung cancer (NSCLC) is the leading cause of cancer deaths worldwide. At diagnosis, almost 70% of persons with NSCLC have locally advanced or metastatic disease. Persons with NSCLC are often treated with therapeutic platinum compounds (e.g. cisplatin, carboplatin or oxaliplatin) that cause inter-strand cross-links in DNA. Among individuals with NSCLC, low expression of BRCA1 in the primary tumor correlated with improved survival after platinum-containing chemotherapy.[124][125] This correlation implies that low BRCA1 in cancer, and the consequent low level of DNA repair, causes vulnerability of cancer to treatment by the DNA cross-linking agents. High BRCA1 may protect cancer cells by acting in a pathway that removes the damages in DNA introduced by the platinum drugs. Thus the level of BRCA1 expression is a potentially important tool for tailoring chemotherapy in lung cancer management.[124][125]
Level of BRCA1 expression is also relevant to ovarian cancer treatment. Patients having sporadic ovarian cancer who were treated with platinum drugs had longer median survival times if their BRCA1 expression was low compared to patients with higher BRCA1 expression (46 compared to 33 months).[126]
Patents, enforcement, litigation, and controversy
A patent application for the isolated BRCA1 gene and cancer promoting mutations discussed above, as well as methods to diagnose the likelihood of getting breast cancer, was filed by the University of Utah, National Institute of Environmental Health Sciences (NIEHS) and
The patents began to expire in 2014.According to an article published in the journal, Genetic Medicine, in 2010, "The patent story outside the United States is more complicated.... For example, patents have been obtained but the patents are being ignored by provincial health systems in Canada. In Australia and the UK, Myriad's licensee permitted use by health systems but announced a change of plans in August 2008. Only a single mutation has been patented in Myriad's lone European-wide patent, although some patents remain under review of an opposition proceeding. In effect, the United States is the only jurisdiction where Myriad's strong patent position has conferred sole-provider status."[128][129] Peter Meldrum, CEO of Myriad Genetics, has acknowledged that Myriad has "other competitive advantages that may make such [patent] enforcement unnecessary" in Europe.[130]
As with any gene, finding variation in BRCA1 is not hard. The real value comes from understanding what the clinical consequences of any particular variant are. Myriad has a large, proprietary database of such genotype-phenotype correlations. In response, parallel open-source databases are being developed.
Legal decisions surrounding the BRCA1 and BRCA2 patents will affect the field of genetic testing in general.[131] A June 2013 article, in Association for Molecular Pathology v. Myriad Genetics (No. 12-398), quoted the US Supreme Court's unanimous ruling that, "A naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated," invalidating Myriad's patents on the BRCA1 and BRCA2 genes. However, the Court also held that manipulation of a gene to create something not found in nature could still be eligible for patent protection.[132] The Federal Court of Australia came to the opposite conclusion, upholding the validity of an Australian Myriad Genetics patent over the BRCA1 gene in February 2013.[133] The Federal Court also rejected an appeal in September 2014.[134] Yvonne D'Arcy won her case against US-based biotech company Myriad Genetics in the High Court of Australia. In their unanimous decision on October 7, 2015, the "high court found that an isolated nucleic acid, coding for a BRCA1 protein, with specific variations from the norm that are indicative of susceptibility to breast cancer and ovarian cancer was not a 'patentable invention.'"[135]
Interactions
BRCA1 has been shown to interact with the following proteins:
- ABL1[136]
- AKT1[137][138]
- AR[139]
- ATR[140][141][142][143]
- ATF1[146]
- BACH1[147]
- BARD1[30][40][44][147]
- BRCA2[148][149][150][151]
- BRCC3[148]
- BRE[148]
- BRIP1[34][152][153][154][155][156]
- C-jun[157]
- CHEK2[158][159]
- CLSPN[160]
- COBRA1[161]
- CREBBP[162][163][164][165][166]
- CSNK2B[167]
- CSTF2[168][169]
- CDK2[170][171][172]
- ELK4[175]
- EP300[163][165]
- ESR1[165][176][177][178]
- FANCA[179]
- FANCD2[180][150]
- FHL2[181][182]
- H2AFX[183][184][185]
- JUNB[157]
- JunD[157]
- LMO4[186][187]
- MAP3K3[188]
- MED1[153]
- MED17[189][153][190]
- MED21[191]
- MED24[153]
- MRE11A[16][189][192][193]
- MSH2[16][40]
- MSH3[40][152]
- MSH6[16][40]
- Myc[44][194][195][196]
- NBN[16][189][192]
- NMI[194]
- NPM1[197]
- NCOA2[152][198]
- NUFIP1[199]
- P53[148][164][200][201][202]
- PALB2[203]
- POLR2A[189][191][204][205]
- PPP1CA[206]
- Rad50[16][189][192]
- RAD51[40][148][149][207]
- RBBP4[208]
- RBBP7[208][209][210]
- RBBP8[211][152][212][213][214][215][216]
- RELA[162]
- RB1[208][217][218]
- RBL1[217]
- RBL2[217]
- RPL31[210]
- SMARCA4[219][220]
- SMARCB1[219]
- STAT1[221]
- TOPBP1[222]
- UBE2D1[183][223][224][225][184][148][197][180][226][227]
- USF2[228]
- VCP[229]
- ZNF350[232]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000012048 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000017146 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Hamel PJ (2007-05-29). "BRCA1 and BRCA2: No Longer the Only Troublesome Genes Out There". HealthCentral. Retrieved 2010-07-02.
- ^ a b "BRCA1 gene tree". Ensembl.
- PMID 10193517.
- S2CID 24297965.
- ^ Check W (September 2006). "BRCA: What we know now". College of American Pathologists. Retrieved 2010-08-23.
- ^ PMID 26738429.
- PMID 17683622.
- ^ Friedenson B (2008-06-08). "Breast cancer genes protect against some leukemias and lymphomas" (video). SciVee.
- ^ "Breast and Ovarian Cancer Genetic Screening". Palo Alto Medical Foundation. Archived from the original on 4 October 2008. Retrieved 2008-10-11.
- ^ PMID 17683622.
- PMID 20400477.
- ^ PMID 10783165.
- ^ PMID 12787778.
- ^ a b c US patent 5747282, Skolnick HS, Goldgar DE, Miki Y, Swenson J, Kamb A, Harshman KD, Shattuck-Eidens DM, Tavtigian SV, Wiseman RW, Futreal PA, "7Q-linked breast and ovarian cancer susceptibility gene", issued 1998-05-05, assigned to Myriad Genetics, Inc., The United States of America as represented by the Secretary of Health and Human Services, and University of Utah Research Foundation
- ^ a b US patent 5837492, Tavtigian SV, Kamb A, Simard J, Couch F, Rommens JM, Weber BL, "Chromosome 13-linked breast cancer susceptibility gene", issued 1998-11-17, assigned to Myriad Genetics, Inc., Endo Recherche, Inc., HSC Research & Development Limited Partnership, Trustees of the University of Pennsylvania
- ^ a b Myriad Investor Page—see "Myriad at a glance" Archived 2012-10-18 at the Wayback Machine accessed October 2012
- ^ a b c Schwartz J (2009-05-12). "Cancer Patients Challenge the Patenting of a Gene". The New York Times. Health.
- PMID 2270482.
- ^ High-Impact Science: Tracking down the BRCA genes (Part 1) Archived 2016-02-20 at the Wayback Machine – Cancer Research UK science blog, 2012
- PMID 7545954.
- ^ National Center for Biotechnology Information, U.S. National Library of Medicine EntrezGene reference information for BRCA1 breast cancer 1, early onset (Homo sapiens)
- PMID 9553742.
- S2CID 10138907.
- ^ Universal protein resource accession number P38398 for "Breast cancer type 1 susceptibility protein" at UniProt.
- ^ PMID 22737296.
- ^ S2CID 37617901.
- S2CID 20552445.
- ^ S2CID 19275284.
- PMID 10946236.
- ^ PMID 11877378.
- PMID 25472942.
- ^ "Kimball's Biologh Pages". Archived from the original on 2018-02-12. Retrieved 2010-02-25.
- PMID 23269703.
- PMID 17052168.
- PMID 22193408.
- ^ PMID 11498787.
- PMID 22366898.
- S2CID 22109822.
- PMID 10855792.
- ^ PMID 9788437.
- PMID 11353843.
- PMID 16103751.
- ^ PMID 11739404.
- PMID 18056434.
- PMID 25833843.
- PMID 9159119.
- PMID 10943845.
- PMID 20301425.
- ^ "Genetics". Breastcancer.org. 2012-09-17.
- PMID 16397213.
- S2CID 32023181.
- S2CID 11583160.
- PMID 12670888.
- S2CID 24737909.
- S2CID 2393567.
- S2CID 35616414.
- PMID 19048628.
- PMID 22972572.
- S2CID 7988460.
- PMID 12559046.
- ^ PMID 17412712.
- ^ PMID 24168967.
- PMID 22790015.
- S2CID 22688947.
- ^ Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem. 2000 Oct 27;275(43):33487-96. doi: 10.1074/jbc.M005824200. PMID: 10938285
- ^ a b Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002 Jun;511(2):145-78. doi: 10.1016/s1383-5742(02)00009-1. PMID: 12052432
- PMID 9653432.
- PMID 24240112.
- PMID 30579323.
- PMID 10749912.
- ^ PMID 21195000.
- PMID 23249749.
- PMID 21472990.
- ^ S2CID 206325689.
- PMID 18403632.
- PMID 18082599.
- PMID 18704159.
- PMID 17616978.
- ^ S2CID 23327569.
- ^ S2CID 21387351.
- ^ PMID 7611288.
- S2CID 30686068.
- PMID 10612815.
- ^ PMID 11250694.
- S2CID 22970255.
- PMID 26577449.
- PMID 9663595.
- ^ PMID 9150151.
- PMID 10595255.
- S2CID 13028232.
- PMID 11597388.
- PMID 9585608.
- S2CID 41804152.
- S2CID 24615109.
- S2CID 23931343.
- PMID 10053113.
- ^ "Mutation data of the BRCA1 gene". KMDB/MutationView (Keio Mutation Databases). Keio University.
- PMID 12142080.
- S2CID 25394976.
- S2CID 2995.
- PMID 11595708.
- S2CID 37710898.
- ^ PMID 12698193.)
{{cite journal}}: CS1 maint: numeric names: authors list (link - PMID 10595257.
- PMID 14522380.
- PMID 12181777.
- PMID 10788334.
- S2CID 7001156.
- PMID 9150173.
- PMID 10682686.
- S2CID 39462456.
- S2CID 34333797.
- PMID 11781691.
- PMID 18192670.
- PMID 19881348.
- ^ PMID 19996028.
- S2CID 19572648.
- ^ PMID 23408054.
- ^ Bernstein H; Hopf FA; Michod RE (1987). The Molecular Basis of the Evolution of Sex. Adv. Genet. Advances in Genetics. Vol. 24. pp. 323–70. doi:10.1016/S0065-2660(08)60012-7. ISBN 9780120176243. PMID 3324702
- ^ PMID 15317748.
- ^ PMID 22425915.
- S2CID 13357407.
- ^ "ACLU sues over patents on breast cancer genes". CNN. Archived from the original on 15 May 2009. Retrieved 2009-05-14.
- PMID 20393305.
- PMID 12509391.
- ^ Conley J, Vorhous D, Cook-Deegan J (March 2011). "How Will Myriad Respond to the Next Generation of BRCA Testing?". Robinson, Bradshaw, and Hinson. Retrieved 2012-12-09.
- ^ "Genetics and Patenting". Human Genome Project Information. U.S. Department of Energy Genome Programs. 2010-07-07.
- ^ Liptak A (June 13, 2013). "Supreme Court Rules Human Genes May Not Be Patented". The New York Times. Retrieved June 13, 2013.
- Sydney Morning Herald. Retrieved June 14, 2013.
- ^ "Australian federal court rules isolated genetic material can be patented". The Guardian. 5 September 2014. Retrieved 14 September 2014.
- ^ "Patient wins high court challenge against company's cancer gene patent". The Guardian. 7 October 2015. Retrieved 6 October 2015.
- PMID 12024016.
- PMID 10542266.
- PMID 19074868.
- PMID 11016951.
- ^ PMID 10608806.
- ^ PMID 11114888.
- ^ PMID 11016625.
- ^ PMID 11278964.
- PMID 10866324.
- PMID 10550055.
- PMID 10945975.
- ^ PMID 11301010.
- ^ PMID 14636569.
- ^ PMID 9774970.
- ^ PMID 14499622.
- PMID 11477095.
- ^ PMID 14578343.
- ^ PMID 15208681.
- PMID 15242590.
- S2CID 29407635.
- S2CID 7354915.
- ^ PMID 12080089.
- S2CID 4345911.
- PMID 18804494.
- PMID 15096610.
- PMID 11739404.
- ^ PMID 12700228.
- ^ PMID 10655477.
- ^ PMID 9926942.
- ^ PMID 11782371.
- PMID 9443979.
- PMID 10403822.
- PMID 11257228.
- PMID 10477523.
- PMID 9244350.
- PMID 8764100.
- PMID 10373534.
- PMID 12592385.
- S2CID 10953768.
- PMID 11313879.
- PMID 11493692.
- PMID 11244506.
- PMID 12400015.
- PMID 12354784.
- ^ PMID 12887909.
- S2CID 31566004.
- PMID 14986435.
- ^ PMID 12485996.
- ^ PMID 11927591.
- PMID 10959836.
- S2CID 20908722.
- PMID 11751867.
- PMID 15205325.
- ^ PMID 11504724.
- PMID 12154023.
- ^ PMID 9159119.
- ^ PMID 10426999.
- PMID 11353843.
- ^ PMID 11916966.
- PMID 14612409.
- PMID 12646176.
- ^ PMID 15184379.
- PMID 11085509.
- PMID 15107825.
- S2CID 20898175.
- PMID 9482880.
- PMID 9582019.
- PMID 19369211.
- PMID 14506230.
- PMID 12955082.
- PMID 12438214.
- PMID 9008167.
- ^ PMID 10220405.
- PMID 11394910.
- ^ S2CID 29703139.
- PMID 9738006.
- PMID 10196224.
- PMID 9811458.
- S2CID 3266654.
- S2CID 4329675.
- PMID 10764811.
- ^ PMID 11521194.
- PMID 10518542.
- ^ PMID 10943845.
- S2CID 40668596.
- PMID 10792030.
- PMID 20012580.
- PMID 12732733.
- PMID 14638690.
- PMID 12438698.
- PMID 12890688.
- PMID 11278247.
- S2CID 24956554.
- PMID 10855792.
- PMID 15065664.
- PMID 12419249.
- PMID 11090615.
External links
- BRCA1 Protein at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Genes, BRCA1 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- PDBe-KB provides an overview of all the structure information available in the PDB for Human BRCA1.