Bacterial cellulose

Bacterial cellulose is an
Historically, bacterial cellulose has been limited to the manufacture of the jelly-like desserts nata de piña and nata de coco, a Filipino food product.[2][3][4] With advances in the ability to synthesize and characterize bacterial cellulose, the material is being used for a wide variety of commercial applications including textiles, cosmetics, and food products, as well as medical applications. Many patents have been issued in microbial cellulose applications and several active areas of research are attempting to better characterize microbial cellulose and utilize it in new areas.[1]
History
As a material, cellulose was first discovered in 1838 by Anselme Payen. Payen was able to isolate the cellulose from the other plant matter and chemically characterize it. In one of its first and most common industrial applications, cellulose from wood pulp was used to manufacture paper. It is ideal for displaying information in print form due to its high reflectivity, high contrast, low cost and flexibility. The discovery of cellulose produced by bacteria, specifically from the
In the mid-1900s, Hestrin et al. proved the necessity of glucose and oxygen in the synthesis of bacterial cellulose. Soon after, Colvin detected cellulose synthesis in samples containing cell-free extract of A. xylinum, glucose and ATP.[8] In 1949, the microfibrillar structure of bacterial cellulose was characterized by Muhlethaler.[9] Further bacterial cellulose studies have led to new uses and applications for the material.
Biosynthesis

Bacterial sources
Bacteria that produce cellulose include
General process
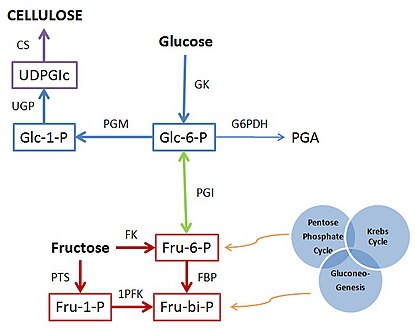
The synthesis of bacterial cellulose is a multistep process that involve two main mechanisms: the synthesis of
Fermentation production
| Microorganism | Carbon source | Supplement | Culture time (h) | Yield (g/L) |
|---|---|---|---|---|
| A. xylinum BRCS | glucose | ethanol, oxygen | 50 | 15.30 |
| G. hansenii PJK (KCTC 10505 BP) | glucose | oxygen | 48 | 1.72 |
| glucose | ethanol | 72 | 2.50 | |
| Acetobacter sp. V6 | glucose | ethanol | 192 | 4.16 |
| Acetobacter sp. A9 | glucose | ethanol | 192 | 15.20 |
| A. xylinum ssp. Sucrofermentans BPR2001 | molasses | none | 72 | 7.82 |
| fructose | agar oxygen | 72 | 14.10 | |
| fructose | agar | 56 | 12.00 | |
| fructose | oxygen | 52 | 10.40 | |
| fructose | agar oxygen | 44 | 8.70 | |
| A. xylinum E25 | glucose | no | 168 | 3.50 |
| G. xylinus K3 | mannitol | green tea | 168 | 3.34 |
| G. xylinus IFO 13773 | glucose | lignosulphonate | 168 | 10.10 |
| A. xylinum NUST4.1 | glucose | sodium alginate | 120 | 6.00 |
| G. xylinus IFO 13773 | sugar cane molasses | no | 168 | 5.76 |
| G. xylinus sp. RKY5 | glycerol | no | 144 | 5.63 |
| Gluconacetobacter sp. St-60-12 and Lactobacillus Mali JCM1116 (co-culture) | sucrose | no | 72 | 4.20 |
Cellulose production depends heavily on several factors such as the
Addition of extra nitrogen generally decreases cellulose production while addition of precursor molecules such as
The other main environmental factors affecting cellulose production are pH, temperature, and dissolved oxygen. According to experimental studies, the optimal temperature for maximum production was between 28 and 30 °C.[27] For most species, the optimal pH was between 4.0 and 6.0.[17] Controlling pH is especially important in static cultures as the accumulation of gluconic, acetic, or lactic acid decreases the pH far lower than the optimal range. Dissolved oxygen content can be varied with stirrer speed as it is needed for static cultures where substrates need to be transported by diffusion.[28]
Reactor based production
Static and agitated cultures are conventional ways to produce bacterial cellulose. Both static and agitated cultures are not feasible for large-scale production as static cultures have a long culture period as well as intensive manpower and agitated cultures produce cellulose-negative mutants alongside its reactions due to rapid growth.[29] Thus, reactors are designed to lessen culture time and inhibit the conversion of bacterial cellulose-producing strains into cellulose-negative mutants. Common reactors used are the rotating disk reactor,[30] the rotary biofilm contactor (RBC),[29] a bioreactor equipped with a spin filter,[31] and a reactor with a silicone membrane.[32]
Structure and properties
| Genus | Cellulose type | Biological role |
|---|---|---|
| Acetobacter | Extracellular pellicle, ribbons |
Maintain aerobic environment |
| Achromobacter | Ribbons | Flocculation |
| Aerobacter | Fibrils | Flocculation |
| Agrobacterium | Short fibrils | Attachment to plants |
| Alcaligenes | Fibrils | Flocculation |
| Pseudomonas | Non-distinct | Flocculation |
| Rhozobium | Short fibrils | Attachment to plants |
| Sarcina | Amorphous | Unknown |
Differences between plant and bacterial cellulose
As the Earth's most common

Macro structure
Cellulose is composed of carbon, oxygen, and hydrogen, and is classified as a polysaccharide, indicating it is a carbohydrate that exhibits polymeric characteristics. Cellulose is composed of straight chain polymers, whose base units of glucose are held together by beta-linkages. The structural role of cellulose in cell walls has been likened to that of the glass strands of fiberglass or to the supporting rods within reinforced concrete.[citation needed] Cellulose fibrils are highly insoluble and inelastic and, because of their molecular configuration, have a tensile strength comparable to that of steel.[citation needed] Consequently, cellulose imparts a unique combination of chemical resilience and mechanical support and flexibility to the tissues in which it resides.[34] Bacterial cellulose, produced by Acetobacter species, displays unique properties, including high mechanical strength, high water absorption capacity, high crystallinity, and an ultra-fine and highly pure fiber network structure.[35] One of the most important features of bacterial cellulose is its chemical purity. In addition to this, bacterial cellulose is stable towards chemicals and at high temperatures.[36] Bacterial cellulose has been suggested to have a construction like a ‘cage' which protects the cell from foreign material and heavy-metal ions, while still allowing nutrients to be supplied easily by diffusion.[2][37] Bacterial cellulose was described by Louis Pasteur as "a sort of moist skin, swollen, gelatinous and slippery." Although the solid portion in the gel is less than one percent, it is almost pure cellulose containing no lignin and other foreign substances.[2] Although bacterial cellulose is obtained in the form of a highly swollen gel, the texture is quite unique and different from typical gels. Cellulose has a high swollen fiber network resulting from the presence of pore structures and tunnels within the wet pellicle. Plant cellulose water retention values 60%, while bacterial cellulose has a water retention value of 1000%.[33] The formation of the cellulose pellicle occurs on the upper surface of the

Properties and characterization
Sheet-shaped material prepared from bacterial cellulose has remarkable mechanical properties. According to Brown, the
This property arises from adjacently aligned glucan chains participating in inter- and intrachain hydrogen bonding.[34] Bacterial cellulose subfibrils are crystallized into microfibrils which group to form bundles, that then form 'ribbons'. These fibers are two orders of magnitude thinner than cellulose fibers produced by pulping wood.[8] Today, it is known that the pellicle comprises a random assembly of fibrils (< 130 nm wide), which are composed of a bundle of much finer microfibrils (2 to 4 nm diameter). It is also known that the pellicle gives a film or sheet when dried if the shrinkage across the plane is restricted.[38] The ultrafine ribbons of microbial cellulose form a dense reticulated structure, stabilized by extensive hydrogen bonding. Bacterial cellulose is also distinguished from its plant counterpart by a high crystallinity index (above 60%). Two common crystalline forms of cellulose, designated as I and II, are distinguishable by X-ray, nuclear magnetic resonance (NMR), Raman spectroscopy, and infrared analysis.[8] Bacterial cellulose belongs crystallographically to Cellulose I, common with natural cellulose of vegetable origin, in which two cellulose units are arranged parallel in a unit cell.[2][40] The term Cellulose I is used for this parallel arrangement, whereas crystalline fibrils bearing antiparallel
Although cellulose forms a distinct crystalline structure, cellulose fibers in nature are not purely crystalline. In addition to the crystalline and
Scanning electron microscopy of a fractured edge has revealed a pile of very thin layers. It is suggested that these fibrils in layers are bound through interfibrillar hydrogen bonds, just as in pulp-papers, but the density of the interfibrillar hydrogen bonds must be much higher as the fibrils are finer, hence the contact area is larger.[38]
Applications
Bacterial cellulose has a wide variety of current and potential future applications. Due to its many unique properties, it has been used in the food industry, the medical field, commercial and industrial products, and other technical areas. Bacterial cellulose is a versatile structural material, allowing it to be shaped in a variety of ways to accommodate different uses. A number of
Food
The oldest known use of bacterial cellulose is as the raw material of
Commercial products
Bacterial cellulose also has wide applications in commercial industries. In papermaking, it is used as an ultra-strength paper and as a reticulated fine fibre network with coating, binding, thickening and suspending characteristics.
Medical
In more modern applications, microbial cellulose has become relevant in the
Microbial cellulose has also been used for internal treatments, such as
Current research/future applications
An area of active research on microbial cellulose is in the area of
Challenges/limitations
Due to the inefficient production process, the current price of bacterial cellulose remains too high to make it commercially attractive and viable on a large scale.[35] Traditional production methods cannot produce microbial cellulose in commercial quantities, so further advancements with reactor based production must be achieved to be able to market many microbial cellulose products.[29]
See also
References
- ^ .
- ^ S2CID 81685441.
- S2CID 56381771.
- ISBN 9718538615. Archived(PDF) from the original on 2021-06-28. Retrieved 2021-03-07.
- ^ Brown, A.J. J. Chem. Soc.,49,172, 432(1886);51,643(1887)
- .
- ^ Tarr, H.L.A., Hibbery, H. Can. J. Research, 4, 372 (1931)
- ^ a b c d A. Steinbuhel, "Bacterial Cellulose." Biopolymers. Weinheim: Wiley-VCH, 2001. Print.
- ^ a b c Bajaj, I; Chawla, P; Singhal, R; Survase, S. "Microbial cellulose: fermentative production and applications". Food Technology and Biotechnology. 47 (2): 107–124.
- .
- ^ S. Bielecki, A. Krystynowicz, M. Turkiewicz, H. Kalinowska: Bacterial Cellulose. In: Polysaccharaides and Polyamides in the Food Industry, A. Steinbuchel, S.K. Rhee (Eds.), Wiley-VCH Verlag, Weinhein, Germany (2005) pp. 31-85
- ^ .
- PMID 7640530.
- ^ .
- PMID 26958837.
- S2CID 83658095.
- ^ .
- S2CID 6660565.
- .
- .
- PMID 15712301.
- .
- PMID 12688462.
- .
- S2CID 33497254.
- PMID 17440758.
- PMID 13208601.
- PMID 7727342.
- ^ S2CID 38869200.
- S2CID 505777.
- S2CID 56424486.
- .
- ^ .
- ^ PMID 2030672.
- ^ .
- PMID 20644807.
- ^ PMID 12209002.
- ^ S2CID 135518566.
- .
- .
- PMID 14546639.
- ^ Okiyama, A., Motoki, M. and Yamanaka, S., Food Hydeocoll., 1992, 6, 479.
- ^ PMID 17206781.
- S2CID 97758926.
- S2CID 25566915.
