Pathogenic bacteria
| Pathogenic bacteria | |
|---|---|
 | |
| Neisseria gonorrhoeae (small red dots) in pus from a man with a urethral discharge (Gram stain) |
Pathogenic bacteria are
The body is continually exposed to many species of bacteria, including beneficial
Pathogenic bacteria are specially adapted and endowed with mechanisms for overcoming the normal body defences, and can invade parts of the body, such as the blood, where bacteria are not normally found. Some pathogens invade only the surface

Caused by Mycobacterium tuberculosis bacteria, one of the diseases with the highest disease burden is tuberculosis, which killed 1.4 million people in 2019, mostly in sub-Saharan Africa.[4] Pathogenic bacteria contribute to other globally important diseases, such as pneumonia, which can be caused by bacteria such as Staphylococcus, Streptococcus and Pseudomonas, and foodborne illnesses, which can be caused by bacteria such as Shigella, Campylobacter, and Salmonella. Pathogenic bacteria also cause infections such as tetanus, typhoid fever, diphtheria, syphilis, and leprosy.
Pathogenic bacteria are also the cause of high
Most pathogenic bacteria can be grown in cultures and identified by Gram stain and other methods. Bacteria grown in this way are often tested to find which antibiotics will be an effective treatment for the infection. For hitherto unknown pathogens, Koch's postulates are the standard to establish a causative relationship between a microbe and a disease.
Diseases
Each species has specific effect and causes symptoms in people who are infected. Some people who are infected with a pathogenic bacteria do not have symptoms. Immunocompromised individuals are more susceptible to pathogenic bacteria.[7]
Pathogenic susceptibility
Some pathogenic bacteria cause disease under certain conditions, such as entry through the skin via a cut, through sexual activity or through compromised immune function.[citation needed]

Some species of
Other bacteria are opportunistic pathogens and cause disease mainly in people with immunosuppression or cystic fibrosis. Examples of these opportunistic pathogens include Pseudomonas aeruginosa, Burkholderia cenocepacia, and Mycobacterium avium.[10][11]
Intracellular
Other groups of intracellular bacterial pathogens include Salmonella, Neisseria, Brucella, Mycobacterium, Nocardia, Listeria, Francisella, Legionella, and Yersinia pestis. These can exist intracellularly, but can exist outside host cells.[citation needed]
Infections in specific tissue
Bacterial pathogens often cause infection in specific areas of the body. Others are generalists.
- Lactobacilli species that maintain healthy vaginal microbial populations.[13]
- Bacterial meningitis is a bacterial inflammation of the meninges, which are the protective membranes covering the brain and spinal cord.
- Bacterial pneumonia is a bacterial infection of the lungs.
- gut flora. But a different strain of the same species may be pathogenic. The distinction is sometimes difficult as in the case of Escherichia.
- Bacterial skin infections include:
- Impetigo is a highly contagious bacterial skin infection commonly seen in children.[17] It is caused by Staphylococcus aureus, and Streptococcus pyogenes.[18]
- Erysipelas is an acute streptococcus bacterial infection[19] of the deeper skin layers that spreads via with lymphatic system.
- intravenous catheterinsertion. In most cases it is the skin on the face or lower legs that is affected, though cellulitis can occur in other tissues.
Mechanisms of damage
The symptoms of disease appear as pathogenic bacteria damage host tissues or interfere with their function. The bacteria can damage host cells directly or indirectly by provoking an immune response that inadvertently damages host cells,[21] or by releasing toxins.[22]
Direct
Once pathogens attach to host cells, they can cause direct damage as the pathogens use the host cell for nutrients and produce waste products.
Toxin production

Indirect
An excessive or inappropriate immune response triggered by an infection may damage host cells.[1]
Survival in host
Nutrients
Iron is required for humans, as well as the growth of most bacteria. To obtain free iron, some pathogens secrete proteins called
Bacterial pathogens also require access to carbon and energy sources for growth. To avoid competition with host cells for glucose which is the main energy source used by human cells, many pathogens including the respiratory pathogen Haemophilus influenzae specialise in using other carbon sources such as lactate that are abundant in the human body [26]
Identification
Typically identification is done by growing the organism in a wide range of cultures which can take up to 48 hours. The growth is then visually or genomically identified. The cultured organism is then subjected to various assays to observe reactions to help further identify species and strain.[27]
Treatment
Bacterial infections may be treated with
Prevention
Infections can be prevented by
List of genera and microscopy features
Many
| Genus | Species | Gram staining
|
Shape | Oxygen requirement | Intra/Extracellular |
|---|---|---|---|---|---|
| Bacillus[31] | Positive | Rods | Facultative anaerobic | Extracellular | |
| Bartonella[31] | Negative | Rods | Aerobic | Facultative intracellular | |
| Bordetella[31] | Negative | Small coccobacilli
|
Aerobic | Extracellular | |
| Borrelia[31] | Negative, stains poorly | Spirochete
|
Anaerobic | Extracellular | |
| Brucella[31] | Negative | Coccobacilli
|
Aerobic | Intracellular | |
| Campylobacter[31] | Negative | Spiral rods[34] coccoid in older cultures[34] |
Microaerophilic[34]
|
Extracellular | |
Chlamydia and Chlamydophila[31]
|
|
(not Gram-stained) | Small, round, ovoid | Facultative or strictly aerobic | Obligate intracellular |
| Clostridium[31] |
|
Positive | Large, blunt-ended rods | Obligate anaerobic | Extracellular |
| Corynebacterium[31] | Positive (unevenly) | Rods | Mostly facultative anaerobic | Extracellular | |
| Enterococcus[33][37] | Positive | Cocci | Facultative Anaerobic | Extracellular | |
| Escherichia[5][33][38] | Negative | Rods | Facultative anaerobic | Extracellular or Intracellular | |
| Francisella[31] | Negative | Coccobacillus | Strictly aerobic | Facultative intracellular | |
| Haemophilus | Negative | Coccobacilli to long and slender filaments | Facultative anaerobic 5 - 10% CO2 | Extracellular | |
| Helicobacter | Negative | Spiral rod | Microaerophile | Extracellular | |
| Legionella[31] | Negative, stains poorly | Cocobacilli | Aerobic | Facultative intracellular | |
| Leptospira[33][41] | Negative, stains poorly | Spirochete
|
Strictly aerobic | Extracellular | |
| Listeria[31] | Positive, darkly | Slender, short rods | Facultative Anaerobic | Facultative intracellular | |
| Mycobacterium[31] | (none) | Long, slender rods | Aerobic | Intracellular | |
| Mycoplasma[31] | (none) | Indistinct 'fried egg' appearance, no cell wall | Mostly facultative anaerobic; M. pneumoniae strictly aerobic | Extracellular | |
| Neisseria[33][42] | Negative | Kidney bean-shaped | Aerobic | Gonococcus: facultative intracellular N. meningitidis: extracellular | |
| Pseudomonas[33][43] | Negative | Rods | Obligate aerobic | Extracellular | |
| Rickettsia[31] | Negative, stains poorly | Small, rod-like coccobacillary | Aerobic | Obligate intracellular | |
| Salmonella[31] |
|
Negative | Rods | Facultative anaerobica | Facultative intracellular |
| Shigella[33][44] | Negative | Rods | Facultative anaerobic | Extracellular | |
| Staphylococcus[5] | Positive, darkly | Round cocci | Facultative anaerobic | Extracellular, facultative intracellular | |
| Streptococcus[31] | Positive | Ovoid to spherical | Facultative anaerobic | Extracellular | |
| Treponema[31] | Negative, stains poorly | Spirochete | Aerobic | Extracellular | |
| Ureaplasma[5] | Stains poorly[45] | Indistinct, 'fried egg' appearance, no cell wall | Anaerobic | Extracellular | |
| Vibrio[33][46] | Negative | Spiral with single polar flagellum | Facultative anaerobic | Extracellular | |
| Yersinia[33][47] | Negative, bipolarly | Small rods | Facultative anaerobe | Intracellular |
List of species and clinical characteristics
-
Overall age-standardised mortality rate per 100 000 population for 33 pathogens investigated, 2019[3]
-
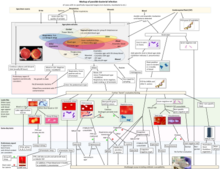
Global number of deaths (A) and YLLs (B), by pathogen and infectious syndrome, 2019[3]
-
Global number of deaths, by pathogen, age, and sex groups, 2019[3]
This is description of the more common genera and species presented with their clinical characteristics and treatments.
| Species | Transmission
|
Diseases | Treatment | Prevention | ||
|---|---|---|---|---|---|---|
| Actinomyces israelii | Oral flora[48]
|
Prolonged | ||||
| Bacillus anthracis |
Contact with cattle, sheep, goats and horses[51] |
Anthrax: pulmonary, gastrointestinal and/or cutaneous symptoms.[48] |
In early infection:[52] |
Autoclaving of equipment[33]
| ||
| Bacteroides fragilis | Gut flora[48]
|
Abscesses in gastrointestinal tract, pelvic cavity and lungs[48] | metronidazole[48] | Wound care[54] | ||
| Bordetella pertussis |
Contact with respiratory droplets expelled by infected human hosts.[33] |
Macrolides[33] such as erythromycin,[33][48] before paroxysmal stage[48] |
Pertussis vaccine,[33][48] such as in DPT vaccine[33][48] | |||
| Borrelia | B. burgdorferi[33][48] |
Ixodes hard ticks |
|
Doxycycline for adults, amoxicillin for children, ceftriaxone for neurological involvement[56] |
Wearing clothing that limits skin exposure to ticks.[33] | |
B. recurrentis[58] and others[note 1] |
Pediculus humanus corporis body louse (B. recurrentis only) and Ornithodoros soft ticks[58]
|
Relapsing fever | Penicillin, tetracycline, doxycycline[59] | Avoid areas where ticks are found[58] Better access to washing facilities[58] | ||
| Brucella | B. abortus |
Direct contact with infected animal[33] |
night sweats
|
|||
| Campylobacter jejuni |
Fecal–oral from animals (mammals and fowl)[33][48] |
|
Treat symptoms[33] |
Good hygiene[33] Pasteurizing milk and milk products[33] Cooking meat (especially poultry)[33] | ||
| Chlamydia | C. pneumoniae | Atypical pneumonia[48] | None[33] | |||
| C. trachomatis |
vaginal sex[33] oral sex[33] anal sex[33] Vertical from mother to newborn( ICN)[33] Direct or contaminated surfaces and flies (trachoma)[33] |
Trachoma[33][48] Urethritis[33][48] Pelvic inflammatory disease[33][48] Epididymitis[33][48] Prostatitis[33][48] Lymphogranuloma venereum (LGV)[33][48] |
Erythromycin[33][48] |
Erythromycin or newborn's eyes[33] Safe sex[33] Abstinence[33] | ||
Chlamydophila psittaci
|
Inhalation of dust with secretions or feces from birds (e.g. parrots) | Psittacosis, mainly atypical pneumonia | - | |||
| Clostridium | C. botulinum | Spores from soil,[33][48] persevere in canned food, smoked fish and honey[48] |
Botulism: Mainly muscle weakness and paralysis[48] |
Antitoxin[33][48] |
Proper food preservation techniques | |
C. difficile
|
Discontinuing responsible antibiotic[33][48] |
Fecal bacteriotherapy
| ||||
| C. perfringens |
gut flora[33]
|
Gas gangrene[33][48] Acute |
Gas gangrene:
Debridement or amputation[33][48] Food poisoning: Supportive care is sufficient[33] |
Appropriate food handling[33] | ||
| C. tetani |
muscle spasms[60]
|
Sedatives[33] Muscle relaxants[33] Mechanical ventilation[33][48] Penicillin or metronidazole[48] |
Tetanus vaccine (such as in the DPT vaccine)[33] | |||
| Corynebacterium diphtheriae |
respiratory droplets |
Diphtheria: Fever, sore throat and neck swelling, potentially narrowing airways.[61] |
Horse serum antitoxin |
|||
| Ehrlichia | E. canis[48] |
Dog tick[48] | fatigue
|
| ||
| Enterococcus | E. faecalis |
Part of |
Bacterial endocarditis,[48] biliary tract infections,[48] urinary tract infections[48] |
Ampicillin (combined with aminoglycoside in endocarditis)[48] Vancomycin[33] |
No vaccine Hand washing and other nosocomial prevention
| |
| Escherichia | E. coli (generally) |
|
UTI:[33]
(resistance-tests are required first)
Meningitis:[33]
Diarrhea:[33]
|
(no vaccine or preventive drug)[33] | ||
| Enterotoxigenic E. coli (ETEC) |
|
| ||||
Enteropathogenic E. coli
|
|
| ||||
| Enteroinvasive E.coli (EIEC) |
| |||||
Enterohemorrhagic (EHEC), including E. coli O157:H7
|
|
|||||
| Francisella tularensis | Tularemia: Fever, ulceration at entry site and/or lymphadenopathy.[63] Can cause severe pneumonia.[63] | |||||
| Haemophilus influenzae | Meningitis:[33]
(resistance-tests are required first)
|
| ||||
| Helicobacter pylori |
|
|
(No vaccine or preventive drug)[33] | |||
| Klebsiella pneumoniae |
|
|
|
|||
| Legionella pneumophila |
|
|
(no vaccine or preventive drug)[33]
Heating water[33] | |||
| Leptospira species |
|
|
|
Vaccine not widely used[33]
Prevention of exposure[33]
| ||
| Listeria monocytogenes | (no vaccine)[33]
| |||||
| Mycobacterium | M. leprae
|
|
|
Tuberculoid form:
Lepromatous form: |
| |
M. tuberculosis
|
|
|
(difficult, see Tuberculosis treatment for more details)[33]
Standard "short" course:[33]
|
| ||
| Mycoplasma pneumoniae | ||||||
| Neisseria | N. gonorrhoeae
|
|
Uncomplicated gonorrhea:[33]
Ophthalmia neonatorum: |
(No vaccine)[33]
| ||
N. meningitidis
|
|
|
| |||
| Pseudomonas aeruginosa | Opportunistic;[48] Infects damaged tissues or people with immunodeficiency.[33] | Pseudomonas infection:[33] | (no vaccine)[33]
| |||
| Nocardia asteroides | In soil[48] | Nocardiosis:[48] Pneumonia, endocarditis, keratitis, neurological or lymphocutaneous infection | TMP/SMX[48]
|
|||
| Rickettsia rickettsii |
|
(no preventive drug or approved vaccine)[33]
| ||||
| Salmonella | S typhi
|
|
|
|||
| Other Salmonella species
|
|
(No vaccine or preventive drug)[33] | ||||
| Shigella | S. sonnei[33] |
|
|
|||
| Staphylococcus | aureus |
|
Coagulase-positive staphylococcal infections:
|
|
(no vaccine or preventive drug)
| |
| epidermidis | Human flora in skin, mucous membranes[48]
|
|
None[33] | |||
| saprophyticus | Part of normal vaginal flora[33] |
|
|
None[33] | ||
| Streptococcus | agalactiae | Human flora in vagina,[33][48] urethral mucous membranes,[33] rectum[33]
|
|
|
None[33] | |
| pneumoniae |
|
|
|
|||
| pyogenes |
|
|
No vaccine[33]
| |||
viridans
|
Oral flora,[48] penetration through abrasions
|
|
Penicillin G[48]
|
|||
| Treponema pallidum subspecies pallidum |
|
|
||||
| Vibrio cholerae |
|
|
| |||
| Yersinia pestis | Plague: |
|
||||
Genetic transformation
Of the 59 species listed in the table with their clinical characteristics, 11 species (or 19%) are known to be capable of natural genetic transformation.[81] Natural transformation is a bacterial adaptation for transferring DNA from one cell to another. This process includes the uptake of exogenous DNA from a donor cell by a recipient cell and its incorporation into the recipient cell's genome by recombination. Transformation appears to be an adaptation for repairing damage in the recipient cell's DNA. Among pathogenic bacteria, transformation capability likely serves as an adaptation that facilitates survival and infectivity.[81] The pathogenic bacteria able to carry out natural genetic transformation (of those listed in the table) are Campylobacter jejuni, Enterococcus faecalis, Haemophilus influenzae, Helicobacter pylori, Klebsiella pneumoniae, Legionella pneumophila, Neisseria gonorrhoeae, Neisseria meningitidis, Staphylococcus aureus, Streptococcus pneumoniae and Vibrio cholerae.[citation needed]
See also
- Human microbiome project
- List of antibiotics
- Pathogenic viruses
Notes
- ISBN 978-1-259-83597-1.
References
- ^ ISBN 978-0-07-181826-1.
- S2CID 9273396.
- ^ PMID 36423648.
- ^ "Tuberculosis (TB)". www.who.int.
- ^ PMID 23976885.
- ^ Hou, Chia-Yi (23 November 2022). "Bacterial infections linked to 1 in 8 deaths in 2019". The Hill. Retrieved 12 December 2022.
- PMID 32034433.
- ^ "Streptococcal Infections - Infectious Diseases". MSD Manual Professional Edition. Retrieved 2 May 2021.
- PMID 11885408.
- PMID 7037390.
- PMID 14980298.
- S2CID 45218449.
- PMID 27449868.
- ^ "Urinary Tract Infections". Retrieved 2010-02-04.
- ^ Roxe DM. Urinalysis. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 191. Available from: https://www.ncbi.nlm.nih.gov/books/NBK302/
- S2CID 4724463.
- ^ "Impetigo". National Health Service. 19 October 2017. Page last reviewed: 17/07/2014
- ISBN 978-1-4160-2973-1
- ^ "erysipelas" at Dorland's Medical Dictionary
- ^ "cellulitis" at Dorland's Medical Dictionary
- ISBN 9780702040894.
- PMID 28934339.
- ISBN 978-0-321-92915-0.
- ISBN 978-0-12-397188-3.
- ^ ISBN 978-0-321-73360-3.
- PMID 35085362.
- PMID 22610620.
- PMID 15487937.
- PMID 9835883.
- PMID 22833738.
- ^ ISBN 978-0-7817-8215-9.
- PMID 2906642.
- ^ ISBN 978-0-7817-8215-9.
- ^ PMID 24287853.
- ^ .
- ISBN 978-0-9631172-1-2.
- ^ Rollins, David M. (2000). "BSCI424 Laboratory Media". University of Maryland. Retrieved 2008-11-18.
- ^ Cain, Donna (January 14, 2015). "MacConkey Agar (CCCCD Microbiology". Collin College. Archived from the original on April 26, 2015. Retrieved May 3, 2015.
- PMID 6490866.
- S2CID 24668819.
- PMID 6027998.
- University of Nebraska-Medical Center, Clinical Laboratory Science Program. Retrieved 2015-05-03.
- ^ Allen, Mary E. (2005). "MacConkey Agar Plates Protocols". American Society for Microbiology. Archived from the original on 2015-05-07. Created: 30 September 2005. Last update: 01 April 2013
- ^ "Hektoen Enteric Agar". Austin Community College District. Retrieved 2015-05-03.
- S2CID 6685738.
- S2CID 34004290.
- ^ "Yersinia pestis" (PDF). Wadsworth Center. 2006.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce cf cg ch ci cj ck cl cm cn co cp cq cr cs ct cu cv cw cx cy cz da db dc dd de df dg dh di dj dk dl dm dn do dp dq dr ds dt du dv dw dx dy dz ea eb ec ed ee ef eg eh ei ej ek el em en eo ep eq er es et eu ev ew ex ey ez fa fb fc fd fe ff fg fh fi fj fk fl fm fn fo fp fq fr fs ft fu fv fw fx fy fz ga gb gc gd ge gf gg gh gi gj gk gl gm gn go gp gq gr gs gt gu gv gw gx gy gz ha hb hc hd he hf hg "Bacteria Table" (PDF). Creighton University School of Medicine. Archived from the original (PDF) on 2015-05-01. Retrieved 2015-05-03.
- S2CID 19554893.
- PMID 12662015.
- ^ "Anthrax in animals". Food and Agriculture Organization. 2001.
- ^ "CDC Anthrax Q & A: Treatment". Archived from the original on 5 May 2011. Retrieved 4 April 2011.
- ^ "FDA approves raxibacumab to treat inhalational anthrax". Food and Drug Administration. Retrieved 14 December 2012.
- ^ a b Itzhak Brook (Jan 28, 2014). "Bacteroides Infection Follow-up". Medscape. Retrieved 2015-09-25.
- PMID 24785207.
- ^ PMID 26593256.
- PMID 26593257.
- ^ ISBN 978-1-259-83597-1.
- PMID 26593261.
- ISBN 9780983263135. Archivedfrom the original on 13 February 2015. Retrieved 12 February 2015.
- (PDF) from the original on 6 June 2015.
- ^ "ESCHERICHIA COLI". Public Health Agency of Canada. 2012-04-30. Retrieved 2015-06-02.
- ^ a b "Signs & Symptoms". Centers for Disease Control and Prevention. 13 December 2018. Page last reviewed: October 26, 2015
- ISBN 978-0-8385-8529-0.
- ^ "Klebsiella pneumoniae in Healthcare Settings". Centers for Disease Control and Prevention. 19 February 2021. Page last reviewed: November 24, 2010. Page last updated: August 27, 2012
- PMID 20628664.
- S2CID 220576544.
- ^ ISBN 978-1-259-83597-1.
- ^ "Leprosy Fact sheet N°101". World Health Organization. January 2014. Archived from the original on 2013-12-12.
- ^ "Tuberculosis Fact sheet N°104". WHO. October 2015. Archived from the original on 23 August 2012. Retrieved 11 February 2016.
- ^ Institut Pasteur Press Office - Vaccine against shigellosis (bacillary dysentery):a promising clinical trial Archived 2009-02-25 at the Wayback Machine 15 January 2009. Retrieved on 27 February 2009
- ^ Levinson, W. (2010). Review of Medical Microbiology and Immunology (11th ed.). pp. 94–9.
- ^ "Syphilis - CDC Fact Sheet (Detailed)". CDC. 2 November 2015. Archived from the original on 6 February 2016. Retrieved 3 February 2016.
- S2CID 23899851.
- S2CID 208793678.
- PMID 16182593.
- ^ Wagle PM. (1948). "Recent advances in the treatment of bubonic plague". Indian J Med Sci. 2: 489–94.
- PMID 14774219.
- PMID 1356303.
- PMID 17652523.
- ^ a b Bernstein H, Bernstein C, Michod RE (2018). Sex in microbial pathogens. Infection, Genetics and Evolution volume 57, pages 8-25. https://doi.org/10.1016/j.meegid.2017.10.024
External links
- Bacterial Pathogen Pronunciation by Neal R. Chamberlain, Ph.D. at A.T. Still University
- Pathogenic bacteria genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID

![Overall age-standardised mortality rate per 100 000 population for 33 pathogens investigated, 2019[3]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b2/Overall_age-standardised_mortality_rate_per_100_000_population_for_33_pathogens_investigated%2C_2019.jpg/552px-Overall_age-standardised_mortality_rate_per_100_000_population_for_33_pathogens_investigated%2C_2019.jpg)
![Global number of deaths (A) and YLLs (B), by pathogen and infectious syndrome, 2019[3]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/57/Global_number_of_deaths_%28A%29_and_YLLs_%28B%29%2C_by_pathogen_and_infectious_syndrome%2C_2019.jpg/215px-Global_number_of_deaths_%28A%29_and_YLLs_%28B%29%2C_by_pathogen_and_infectious_syndrome%2C_2019.jpg)
![Global number of deaths, by pathogen, age, and sex groups, 2019[3]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/76/Global_number_of_deaths%2C_by_pathogen%2C_age%2C_and_sex_groups%2C_2019.jpg/284px-Global_number_of_deaths%2C_by_pathogen%2C_age%2C_and_sex_groups%2C_2019.jpg)