Bacteriophage

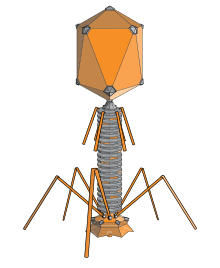

A bacteriophage (
Bacteriophages are among the most common and diverse entities in the
Bacteriophages were used from the 1920s as an alternative to
Bacteriophages are known to interact with the immune system both indirectly via bacterial expression of phage-encoded proteins and directly by influencing innate immunity and bacterial clearance.[13] Phage–host interactions are becoming increasingly important areas of research.[14]
Classification
Bacteriophages occur abundantly in the biosphere, with different genomes and lifestyles. Phages are classified by the International Committee on Taxonomy of Viruses (ICTV) according to morphology and nucleic acid.


| Order | Family | Morphology | Nucleic acid | Examples |
|---|---|---|---|---|
Belfryvirales
|
Turriviridae | Enveloped, isometric | Linear dsDNA | |
| Caudovirales | Ackermannviridae | Nonenveloped, contractile tail | Linear dsDNA | |
| Autographiviridae | Nonenveloped, noncontractile tail (short) | Linear dsDNA | ||
| Chaseviridae | Linear dsDNA | |||
| Demerecviridae | Linear dsDNA | |||
| Drexlerviridae | Linear dsDNA | |||
| Guenliviridae | Linear dsDNA | |||
| Herelleviridae | Nonenveloped, contractile tail | Linear dsDNA | ||
| Myoviridae | Nonenveloped, contractile tail | Linear dsDNA | ||
| Siphoviridae | Nonenveloped, noncontractile tail (long) | Linear dsDNA | N15
| |
| Podoviridae | Nonenveloped, noncontractile tail (short) | Linear dsDNA | P22
| |
| Rountreeviridae | Linear dsDNA | |||
| Salasmaviridae | Linear dsDNA | |||
| Schitoviridae | Linear dsDNA | |||
| Zobellviridae | Linear dsDNA | |||
| Halopanivirales | Sphaerolipoviridae |
Enveloped, isometric | Linear dsDNA | |
Simuloviridae |
Enveloped, isometric | Linear dsDNA | ||
Matshushitaviridae |
Enveloped, isometric | Linear dsDNA | ||
Haloruvirales
|
Pleolipoviridae | Enveloped, pleomorphic | Circular ssDNA, circular dsDNA, or linear dsDNA | |
Kalamavirales
|
Tectiviridae |
Nonenveloped, isometric | Linear dsDNA | |
| Ligamenvirales | Lipothrixviridae | Enveloped, rod-shaped | Linear dsDNA | Acidianus filamentous virus 1
|
| Rudiviridae | Nonenveloped, rod-shaped | Linear dsDNA | Sulfolobus islandicus rod-shaped virus 1
| |
Mindivirales
|
Cystoviridae |
Enveloped, spherical | Linear dsRNA | Φ6 |
| Norzivirales | Atkinsviridae | Nonenveloped, isometric | Linear ssRNA | |
| Duinviridae | Nonenveloped, isometric | Linear ssRNA | ||
| Fiersviridae | Nonenveloped, isometric | Linear ssRNA | Qβ
| |
| Solspiviridae | Nonenveloped, isometric | Linear ssRNA | ||
Petitvirales
|
Microviridae | Nonenveloped, isometric | Circular ssDNA | ΦX174 |
Primavirales
|
Tristromaviridae | Enveloped, rod-shaped | Linear dsDNA | |
| Timlovirales | Blumeviridae | Nonenveloped, isometric | Linear ssRNA | |
| Steitzviridae | Nonenveloped, isometric | Linear ssRNA | ||
| Tubulavirales | Inoviridae |
Nonenveloped, filamentous | Circular ssDNA | M13 |
| Paulinoviridae | Nonenveloped, filamentous | Circular ssDNA | ||
| Plectroviridae | Nonenveloped, filamentous | Circular ssDNA | ||
Vinavirales
|
Corticoviridae |
Nonenveloped, isometric | Circular dsDNA | PM2
|
| Durnavirales | Picobirnaviridae (proposal) |
Nonenveloped, isometric | Linear dsRNA | |
| Unassigned | Ampullaviridae | Enveloped, bottle-shaped | Linear dsDNA | |
| Autolykiviridae | Nonenveloped, isometric | Linear dsDNA | ||
| Bicaudaviridae | Nonenveloped, lemon-shaped | Circular dsDNA | ||
| Clavaviridae | Nonenveloped, rod-shaped | Circular dsDNA | ||
| Finnlakeviridae | Nonenveloped, isometric | Circular ssDNA | FLiP[15]
| |
| Fuselloviridae | Nonenveloped, lemon-shaped | Circular dsDNA | Alphafusellovirus | |
| Globuloviridae | Enveloped, isometric | Linear dsDNA | ||
| Guttaviridae | Nonenveloped, ovoid | Circular dsDNA | ||
| Halspiviridae | Nonenveloped, lemon-shaped | Linear dsDNA | ||
| Plasmaviridae | Enveloped, pleomorphic | Circular dsDNA | ||
| Portogloboviridae | Enveloped, isometric | Circular dsDNA | ||
| Thaspiviridae | Nonenveloped, lemon-shaped | Linear dsDNA | ||
| Spiraviridae | Nonenveloped, rod-shaped | Circular ssDNA |
It has been suggested that members of
There are also many unassigned genera of the class Leviviricetes: Chimpavirus, Hohglivirus, Mahrahvirus, Meihzavirus, Nicedsevirus, Sculuvirus, Skrubnovirus, Tetipavirus and Winunavirus containing linear ssRNA genomes[17] and the unassigned genus Lilyvirus of the order Caudovirales containing a linear dsDNA genome.
History
In 1896, Ernest Hanbury Hankin reported that something in the waters of the Ganges and Yamuna rivers in India had a marked antibacterial action against cholera and it could pass through a very fine porcelain filter.[18] In 1915, British bacteriologist Frederick Twort, superintendent of the Brown Institution of London, discovered a small agent that infected and killed bacteria. He believed the agent must be one of the following:
- a stage in the life cycle of the bacteria
- an enzyme produced by the bacteria themselves, or
- a virus that grew on and destroyed the bacteria[19]
Twort's research was interrupted by the onset of World War I, as well as a shortage of funding and the discoveries of antibiotics.
Independently,
Nobel prizes awarded for phage research
In 1969, Max Delbrück, Alfred Hershey, and Salvador Luria were awarded the Nobel Prize in Physiology or Medicine for their discoveries of the replication of viruses and their genetic structure.[24] Specifically the work of Hershey, as contributor to the Hershey–Chase experiment in 1952, provided convincing evidence that DNA, not protein, was the genetic material of life. Delbrück and Luria carried out the Luria–Delbrück experiment which demonstrated statistically that mutations in bacteria occur randomly and thus follow Darwinian rather than Lamarckian principles.
Uses
Phage therapy

Phages were discovered to be antibacterial agents and were used in the former
- Antibiotics were discovered and marketed widely. They were easier to make, store, and prescribe.
- Medical trials of phages were carried out, but a basic lack of understanding of phages raised questions about the validity of these trials.[26]
- Publication of research in the Soviet Union was mainly in the Russian or Georgian languages and for many years was not followed internationally.
The use of phages has continued since the end of the
Meanwhile, bacteriophage researchers have been developing engineered viruses to overcome antibiotic resistance, and engineering the phage genes responsible for coding enzymes that degrade the biofilm matrix, phage structural proteins, and the enzymes responsible for lysis of the bacterial cell wall.[5][6][7] There have been results showing that T4 phages that are small in size and short-tailed can be helpful in detecting E. coli in the human body.[31]
Therapeutic efficacy of a phage cocktail was evaluated in a mice model with nasal infection of multidrug-resistant (MDR)
D'Herelle "quickly learned that bacteriophages are found wherever bacteria thrive: in sewers, in rivers that catch waste runoff from pipes, and in the stools of convalescent patients."[34] This includes rivers traditionally thought to have healing powers, including India's Ganges River.[35]
Other
Food industry
Phages have increasingly been used to safen food products and to forestall
Water indicators
Bacteriophages, including those specific to Escherichia coli, have been employed as indicators of fecal contamination in water sources. Due to their shared structural and biological characteristics, coliphages can serve as proxies for viral fecal contamination and the presence of pathogenic viruses such as rotavirus, norovirus, and HAV. Research conducted on wastewater treatment systems has revealed significant disparities in the behavior of coliphages compared to fecal coliforms, demonstrating a distinct correlation with the recovery of pathogenic viruses at the treatment's conclusion. Establishing a secure discharge threshold, studies have determined that discharges below 3000 PFU/100 mL are considered safe in terms of limiting the release of pathogenic viruses. [41]
Diagnostics
In 2011, the FDA cleared the first bacteriophage-based product for in vitro diagnostic use.[42] The KeyPath MRSA/MSSA Blood Culture Test uses a cocktail of bacteriophage to detect Staphylococcus aureus in positive blood cultures and determine methicillin resistance or susceptibility. The test returns results in about five hours, compared to two to three days for standard microbial identification and susceptibility test methods. It was the first accelerated antibiotic-susceptibility test approved by the FDA.[43]
Counteracting bioweapons and toxins
Government agencies in the West have for several years been looking to Georgia and the former Soviet Union for help with exploiting phages for counteracting bioweapons and toxins, such as anthrax and botulism.[44] Developments are continuing among research groups in the U.S. Other uses include spray application in horticulture for protecting plants and vegetable produce from decay and the spread of bacterial disease. Other applications for bacteriophages are as biocides for environmental surfaces, e.g., in hospitals, and as preventative treatments for catheters and medical devices before use in clinical settings. The technology for phages to be applied to dry surfaces, e.g., uniforms, curtains, or even sutures for surgery now exists. Clinical trials reported in Clinical Otolaryngology[29] show success in veterinary treatment of pet dogs with otitis.
Bacterium sensing and identification
The sensing of phage-triggered ion cascades (SEPTIC) bacterium sensing and identification method uses the ion emission and its dynamics during phage infection and offers high specificity and speed for detection.[45]
Phage display
Phage display is a different use of phages involving a library of phages with a variable peptide linked to a surface protein. Each phage genome encodes the variant of the protein displayed on its surface (hence the name), providing a link between the peptide variant and its encoding gene. Variant phages from the library may be selected through their binding affinity to an immobilized molecule (e.g., botulism toxin) to neutralize it. The bound, selected phages can be multiplied by reinfecting a susceptible bacterial strain, thus allowing them to retrieve the peptides encoded in them for further study.[46]
Antimicrobial drug discovery
Phage proteins often have antimicrobial activity and may serve as leads for
Basic research
Bacteriophages are important
Detriments
Dairy industry
Bacteriophages present in the environment can cause cheese to not ferment. In order to avoid this, mixed-strain starter cultures and culture rotation regimes can be used.
Some research has focused on the potential of bacteriophages as antimicrobial against foodborne pathogens and biofilm formation within the dairy industry. As the spread of antibiotic resistance is a main concern within the dairy industry, phages can serve as a promising alternative.[52]
Replication

The life cycle of bacteriophages tends to be either a lytic cycle or a lysogenic cycle. In addition, some phages display pseudolysogenic behaviors.[13]
With lytic phages such as the
In contrast, the
Sometimes prophages may provide benefits to the host bacterium while they are dormant by adding new functions to the bacterial
Attachment and penetration
Bacterial cells are protected by a cell wall of
Myovirus bacteriophages use a
Synthesis of proteins and nucleic acid
Within minutes, bacterial
Virion assembly
In the case of the
Release of virions
Phages may be released via cell lysis, by extrusion, or, in a few cases, by budding. Lysis, by tailed phages, is achieved by an enzyme called
Communication
Research in 2017 revealed that the bacteriophage Φ3T makes a short viral protein that signals other bacteriophages to lie dormant instead of killing the host bacterium. Arbitrium is the name given to this protein by the researchers who discovered it.[67][68]
Genome structure
Given the millions of different phages in the environment, phage genomes come in a variety of forms and sizes. RNA phages such as
Some marine roseobacter phages contain deoxyuridine (dU) instead of deoxythymidine (dT) in their genomic DNA. There is some evidence that this unusual component is a mechanism to evade bacterial defense mechanisms such as restriction endonucleases and CRISPR/Cas systems which evolved to recognize and cleave sequences within invading phages, thereby inactivating them. Other phages have long been known to use unusual nucleotides. In 1963, Takahashi and Marmur identified a Bacillus phage that has dU substituting dT in its genome,[74] and in 1977, Kirnos et al. identified a cyanophage containing 2-aminoadenine (Z) instead of adenine (A).[75]
Systems biology
The field of systems biology investigates the complex networks of interactions within an organism, usually using computational tools and modeling.[76] For example, a phage genome that enters into a bacterial host cell may express hundreds of phage proteins which will affect the expression of numerous host genes or the host's metabolism. All of these complex interactions can be described and simulated in computer models.[76]
For instance, infection of Pseudomonas aeruginosa by the temperate phage PaP3 changed the expression of 38% (2160/5633) of its host's genes. Many of these effects are probably indirect, hence the challenge becomes to identify the direct interactions among bacteria and phage.[77]
Several attempts have been made to map protein–protein interactions among phage and their host. For instance, bacteriophage lambda was found to interact with its host, E. coli, by dozens of interactions. Again, the significance of many of these interactions remains unclear, but these studies suggest that there most likely are several key interactions and many indirect interactions whose role remains uncharacterized.[78]
Host resistance
Bacteriophages are a major threat to bacteria and prokaryotes have evolved numerous mechanisms to block infection or to block the replication of bacteriophages within host cells. The CRISPR system is one such mechanism as are retrons and the anti-toxin system encoded by them.[79] The Thoeris defense system is known to deploy a unique strategy for bacterial antiphage resistance via NAD+ degradation.[80]
Bacteriophage–host symbiosis
Temperate phages are bacteriophages that integrate their genetic material into the host as extrachromosomal episomes or as a prophage during a lysogenic cycle.[81][82][83] Some temperate phages can confer fitness advantages to their host in numerous ways, including giving antibiotic resistance through the transfer or introduction of antibiotic resistance genes (ARGs),[82][84] protecting hosts from phagocytosis,[85][86] protecting hosts from secondary infection through superinfection exclusion,[87][88][89] enhancing host pathogenicity,[81][90] or enhancing bacterial metabolism or growth.[91][92][93][94] Bacteriophage–host symbiosis may benefit bacteria by providing selective advantages while passively replicating the phage genome.[95]
In the environment
Metagenomics has allowed the in-water detection of bacteriophages that was not possible previously.[96]
Also, bacteriophages have been used in hydrological tracing and modelling in river systems, especially where surface water and groundwater interactions occur. The use of phages is preferred to the more conventional dye marker because they are significantly less absorbed when passing through ground waters and they are readily detected at very low concentrations.[97] Non-polluted water may contain approximately 2×108 bacteriophages per ml.[98]
Bacteriophages are thought to contribute extensively to
Recent findings have mapped the complex and intertwined arsenal of anti-phage defense tools in environmental bacteria.[101]
In humans
Although phages do not infect humans, there are countless phage particles in the human body, given our extensive microbiome. Our phage population has been called the human phageome, including the "healthy gut phageome" (HGP) and the "diseased human phageome" (DHP).[102] The active phageome of a healthy human (i.e., actively replicating as opposed to nonreplicating, integrated prophage) has been estimated to comprise dozens to thousands of different viruses.[103] There is evidence that bacteriophages and bacteria interact in the human gut microbiome both antagonistically and beneficially.[104]
Preliminary studies have indicated that common bacteriophages are found in 62% of healthy individuals on average, while their prevalence was reduced by 42% and 54% on average in patients with ulcerative colitis (UC) and Crohn's disease (CD).[102] Abundance of phages may also decline in the elderly.[104]
The most common phages in the human intestine, found worldwide, are
Commonly studied bacteriophage
Among the countless phage, only a few have been studied in detail, including some historically important phage that were discovered in the early days of microbial genetics. These, especially the T-phage, helped to discover important principles of gene structure and function.
Bacteriophage databases and resources
See also
- Antibiotic
- Bacterivore
- CrAssphage
- CRISPR
- DNA viruses
- Macrophage
- Phage ecology
- Phage monographs (a comprehensive listing of phage and phage-associated monographs, 1921–present)
- Phagemid
- Polyphage
- RNA viruses
- Transduction
- Viriome
- Virophage, viruses that infect other viruses
References
- S2CID 238939621.
- ^ ISBN 978-1-904455-14-1.
- ^ LaFee S, Buschman H (25 April 2017). "Novel Phage Therapy Saves Patient with Multidrug-Resistant Bacterial Infection". UC Health – UC San Diego. Retrieved 13 May 2018.
- S2CID 4370363.
- ^ PMID 10704475.
- ^ ISBN 0-697-01372-3.
- ^ OCLC 224991186. – Documentary about the history of phage medicine in Russia and the West
- JSTOR 26016042.
- S2CID 73439131.
- PMID 30651225.
- PMID 32899720.
- PMID 22833738.
- ^ PMID 34014761.
- PMID 31216787.
- PMID 28716906.
- PMID 29346073.
- .
- ^ Hankin EH (1896). "L'action bactericide des eaux de la Jumna et du Gange sur le vibrion du cholera". Annales de l'Institut Pasteur (in French). 10: 511–23.
- .
- ^ d'Hérelles F (1917). "Sur un microbe invisible antagoniste des bacilles dysentériques" (PDF). Comptes Rendus de l'Académie des Sciences de Paris. 165: 373–5. Archived (PDF) from the original on 11 May 2011. Retrieved 5 September 2010.
- ^ d'Hérelles F (1949). "The bacteriophage" (PDF). Science News. 14: 44–59. Retrieved 5 September 2010.
- PMID 23231482.
- PMID 34172535.
- ^ "The Nobel Prize in Physiology or Medicine 1969". Nobel Foundation. Retrieved 28 July 2007.
- PMID 30312428.
- S2CID 31626252.
- Наука и жизнь[Nauka I Zhizn (Science and life)] (in Russian) (6): 26–33.
- ^ PMID 19661847.
- ^ PMID 19673983.
- PMID 30923196.
- (PDF) from the original on 2 February 2023.
- PMID 29755420.
- PMID 28807909.
- ISBN 978-1-4614-0250-3
- PMID 19275495.
- ^ S2CID 58620015.
- ^ U.S. FDA/CFSAN: Agency Response Letter, GRAS Notice No. 000198
- ^ (U.S. FDA/CFSAN: Agency Response Letter, GRAS Notice No. 000218)
- ^ "FSIS Directive 7120: Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products" (PDF). Food Safety and Inspection Service. Washington, DC: United States Department of Agriculture. Archived from the original (PDF) on 18 October 2011.
- PMID 37869651.
- ^ Chacón L, Barrantes K, Santamaría-Ulloa C, Solano MReyes L, Taylor LValiente C, Symonds EM, Achí R. 2020. A Somatic Coliphage Threshold Approach To Improve the Management of Activated Sludge Wastewater Treatment Plant Effluents in Resource-Limited Regions. Appl Environ Microbiol 86:e00616-20. https://doi.org/10.1128/AEM.00616-20/
- ^ "FDA 510(k) Premarket Notification". U.S. Food and Drug Administration.
- PMID 32591380. Archived from the originalon 5 April 2024.
- ^ Vaisman D (25 May 2007). "Studying anthrax in a Soviet-era lab – with Western funding". The New York Times.
- doi:10.4024/1050501.jbpc.05.01. Archived from the original(PDF) on 26 September 2018. Retrieved 19 December 2016.
- PMID 11848876.
- S2CID 9905115.
- ^ "Technological background Phage-ligand technology". bioMérieux.
- PMID 24616839.
- PMID 23882262.
- S2CID 7217985.
- PMID 29117107.
- PMID 31623057.
- ISBN 978-0-07-893649-4.
- PMID 19007916.
- S2CID 6907842.
- PMID 23248780.
- PMID 25666799.
- PMID 9356254.
- PMID 31767752.
- S2CID 4289674.
- PMID 30765709.
- PMID 4878023.
- PMID 4907266.
- PMID 18487310.
- S2CID 252542284.
- .
- PMID 28099413.
- PMID 22297527.
- PMID 32051592.
- PMID 22748812.
- PMID 18178732.
- PMID 22126996.
- S2CID 4166988.
- S2CID 4177449.
- ^ OCLC 288986435.
- PMID 26750429.
- PMID 24049175.
- S2CID 250643138.
- PMID 32499527.
- ^ PMID 34071422.
- ^ PMID 33347602.
- PMID 34255542.
- PMID 19858262.
- S2CID 203580138.
- S2CID 204243652.
- PMID 24659988.
- S2CID 7084706.
- OCLC 679550931.
- PMID 15353570.
- S2CID 4156346.
- PMID 25501487.
- PMID 25706310.
- S2CID 3565635.
- PMID 36204422.
- PMID 12384570.
- .
- S2CID 4271861.
- PMID 28096488.
- PMID 27890586.
- PMID 38459056.
- ^ PMID 27573828.
- PMID 21880779.
- ^ PMID 34255542.
- PMID 13978804.
- PMID 12626685.
- S2CID 39369249.
- PMID 37904614.
Bibliography
- Hauser AR, Mecsas J, Moir DT (July 2016). "Beyond Antibiotics: New Therapeutic Approaches for Bacterial Infections". Clinical Infectious Diseases. 63 (1): 89–95. PMID 27025826.
- Strathdee S, Patterson T (2019). The Perfect Predator. ISBN 978-0-316-41808-9.
- Häusler T (2006). Viruses vs. superbugs : a solution to the antibiotics crisis?. London: Macmillan. ISBN 978-1-4039-8764-8.
External links
- Abedon ST. "The Bacteriophage Ecology Group". The Ohio State University. Archived from the original on 3 June 2013.
- Tourterel C, Blouin Y. "Bacteriophages illustrations and genomics". Orsay phage web site. Archived from the original on 29 October 2013. Retrieved 24 October 2013.
- "QuipStories: Bacteriophages get a foothold on their prey" (PDF). PDBe.
- Flatow I (April 2008). "Using 'Phage' Viruses to Help Fight Infection". Science Friday podcast. NPR. Archived from the original on 17 April 2008.
- "Animation of a scientifically correct T4 bacteriophage targeting E. coli bacteria". YouTube.
- "T4 Bacteriophage targeting E. coli bacteria". Animation by Hybrid Animation Medical. 21 December 2009.
- Bacteriophages: What are they. Presentation by Professor Graham Hatfull, University of Pittsburgh on YouTube
