Barium peroxide

| |
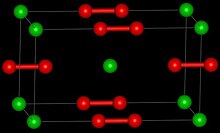 Barium cations Ba2+ Peroxide anions O2−2 | |
| Names | |
|---|---|
| IUPAC name
barium peroxide
| |
| Other names
Barium binoxide,
Barium dioxide | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.013.754 |
| EC Number |
|
PubChem CID
|
|
RTECS number
|
|
| UNII | |
| UN number | 1449 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BaO2 | |
| Molar mass | 169.33 g/mol (anhydrous) 313.45 g/mol (octahydrate) |
| Appearance | Grey-white crystalline solid (anhydrous) Colorless solid (octahydrate) |
| Odor | Odorless |
| Density | 5.68 g/cm3 (anhydrous) 2.292 g/cm3 (octahydrate) |
| Melting point | 450 °C (842 °F; 723 K) |
| Boiling point | 800 °C (1,470 °F; 1,070 K) (decomposes to BaO & O2.[1]) |
| 0.091 g/(100 mL) (20 °C) (anhydrous) 0.168 g/cm3 (octahydrate) | |
| Solubility | dissolves with decomposition in acid |
| −40.6·10−6 cm3/mol | |
| Structure | |
Tetragonal[2]
| |
| D174h, I4/mmm, tI6 | |
| 6 | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H272, H302, H332 | |
| P210, P220, P221, P261, P264, P270, P271, P280, P301+P312, P304+P312, P304+P340, P312, P330, P370+P378, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium peroxide is an
oxidizer and giving a vivid green colour upon ignition (as do all barium compounds), it finds some use in fireworks; historically, it was also used as a precursor for hydrogen peroxide.[3]
Structure
Barium peroxide consists of barium
, CaC2.Preparation and use
Barium peroxide arises by the reversible reaction of O2 with barium oxide. The peroxide forms around 500 °C and oxygen is released above 820 °C.[1]
- 2 BaO + O2 ⇌ 2 BaO2
This reaction is the basis for the now-obsolete Brin process for separating oxygen from the atmosphere. Other oxides, e.g. Na2O and SrO, behave similarly.[4]
In another obsolete application, barium peroxide was once used to produce hydrogen peroxide via its reaction with sulfuric acid:[3]
- BaO2 + H2SO4 → H2O2 + BaSO4
The insoluble barium sulfate is filtered from the mixture.
Footnotes
- ^
- .
- ^ ISBN 978-3527306732.
- ISBN 0-12-352651-5.

