Basal ganglia disease
| Basal ganglia disease | |
|---|---|
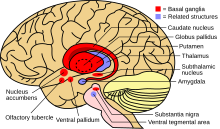 | |
| Basal ganglia and related structures | |
| Specialty | Neurology |
| Types | 8 |
Basal ganglia disease is a group of physical problems that occur when the group of nuclei in the brain known as the
Reasons for abnormal increases or decreases of basal ganglia output are not yet well understood. One possible factor could be the natural accumulation of
Basal ganglia circuits
The basal ganglia is a collective group of structures in the brain. These include the
Understanding these circuits has led to breakthroughs in understanding the disorders of the basal ganglia.Direct pathway
Of all the circuits, the motor circuit is the most studied due its importance to motor disorders. The
Indirect pathway
The
Associated disorders
Hypokinetic disorders
Hypokinetic disorders are movement disorders that are described as having reduced motor function. This is generally attributed to higher than normal basal ganglia output causing inhibition of thalamocortical motor neurons.
Parkinsonism
The muscle rigidity, tremor at rest, and slowness in initiation and execution of movement that are the cardinal motor symptoms of Parkinson's disease are attributed to a reduction in dopaminergic activity in the basal ganglia motor areas, particularly the putamen, due to gradually reduced innervation from the pars compacta of substantia nigra.[6] Other motor deficits and common non-motor features of Parkinson's such as autonomic dysfunction, cognitive impairment, and gait/balance difficulties, are thought to result from widespread progressive pathological changes commencing in the lower brain stem and ascending to the midbrain, amygdala, thalamus and ultimately the cerebral cortex.[4]
Hyperkinetic disorders
Hyperkinetic disorders are movement disorders characterized by increased uncontrollable motor function. They are caused by reduced basal ganglia output, which causes increased thalamocortical function which leads to the inability to stop unwanted movement.
Huntington's disease
Dystonia
Dystonia is a hyperkinetic movement disorder that is characterized by involuntary movement and the slowing of intentional movement. Though there are known causes of dystonia such as metabolic, vascular, and structural abnormalities, there are still patients with dystonia with no apparent cause. Dystonia can occur as a hyperkinetic disorder or as a side effect of hypokinetic disorders such as Parkinson's disease.[9] Until recently it was thought that dystonia was likely caused by extreme lack of function of the direct pathway between the Putamen and the GPi. Again, it was thought that this dysfunction led to a decrease in basal ganglia output to the thalamus and a resultant increased disinhibition of the thalamic projections to the premotor and motor cortex.[10] However recent models in mice show that the dysfunction in the cerebellum may play an equal part in dystonia.[11]
Hemiballismus
Hemiballismus is a hyperkinetic movement disorder that causes uncontrolled movement on one side of the body. It is generally caused by damage to the subthalamic nucleus (STN). Since the internal segment of the globus pallidus (GPi) is the link in the circuit between the STN and thalamic projection, destruction of localized brain cells in the GPi via a pallidotomy has proven to serve as a useful treatment for hemiballismus.[9]
Other basal ganglia diseases
The following diseases that generally involve the basal ganglia do not clearly fit into being either hypo- or hyperkinetic.
Epilepsy
The substantia nigra pars reticulata and its direct input structure, the subthalamic nucleus play a role in seizure propagation circuitry and have been described as seizure gating nuclei. Inhibition of these nuclei suppresses seizures in various experimental epilepsy models. Patients with seizures display some abnormal electrophysiological activity and structural changes like atrophy, altered blood perfusion and metabolism within their basal ganglia. Several case reports describe that deep brain stimulation of the subthalamic nucleus has been successful in reducing seizures. Other targeted treatment approaches have been limited to experimental settings and included local drug infusions and cell transplantation.[12]
Tourette syndrome/obsessive–compulsive disorder
Tourette syndrome is a disorder that is characterized by behavioral and motor tics, OCD and attention deficit hyperactivity disorder (ADHD). For this reason, it is commonly believed that pathologies involving limbic, associative and motor circuits of the basal ganglia are likely. Since the realization that syndromes such as Tourette syndrome and OCD are caused by dysfunction of the non-motor loops of basal ganglia circuits, new treatments for these disorders, based on treatments originally designed to treat movement disorders are being developed.[4]
Sydenham's chorea
Sydenham's chorea is a disorder characterized by rapid, uncoordinated jerking movements primarily affecting the face, hands and feet.
PANDAS
PANDAS is a controversial hypothesis that there exists a subset of children with rapid onset of obsessive–compulsive disorder (OCD) or tic disorders and that these symptoms are caused by group A β-hemolytic streptococcal (GABHS) infections.[17][18][19] The proposed link between infection and these disorders is that an initial autoimmune reaction to a GABHS infection produces antibodies that interfere with basal ganglia function, causing symptom exacerbations. It has been proposed that this autoimmune response can result in a broad range of neuropsychiatric symptoms.[20][21]
Dyskinetic cerebral palsy
Dyskinetic cerebral palsy is a type of
Athymhormic syndrome
Athymhormic syndrome is a rare psychopathological and neurological
Lesch–Nyhan syndrome
Lesch–Nyhan syndrome is a rare
Wilson's disease
Wilson's disease is an
Fahr's disease and calcifications
Blepharospasm
Blepharospasm is any abnormal contraction or twitch of the eyelid. Blepharospasm may come from abnormal functioning of the brain's basal ganglia.[32]
Research
Gene therapy
Many disorders of the basal ganglia are due to the dysfunction of a localized area. For this reason, gene therapy seems viable for neurodegenerative disorders. Gene therapy is performed by replacing diseased phenotypes with new genetic material. This process is still in the early stages but early results are promising. An example of this therapy might involve implanting cells genetically modified to express tyrosine hydroxylase which, in the body, could be converted to dopamine. Increasing dopamine levels in the basal ganglia could possibly offset the effects of the Parkinson's Disease.[1]
Ablation
Lesioning is the intentional destruction of neuronal cells in a particular area used for therapeutic purposes. Though this seems dangerous, vast improvements have been achieved in patients with movement disorders.[33] The exact process generally involves unilateral lesioning in the sensorimotor territory of the GPi. This process is called pallidotomy. It is believed that the success of pallidotomies in reducing the effects of movement disorders may result from the interruption of abnormal neuronal activity in the GPi. This ablation technique can be viewed as simply removing a faulty piece of a circuit. With the damaged piece of the circuit removed, the healthy area of the circuit can continue normal function.[9]
Deep brain stimulation
Deep brain stimulation involves inserting, via stereotaxic surgery, electrodes into the sensorimotor area of the brain.[1][4] These electrodes emit high-frequency stimulation to the implanted areas.[4] Bilateral implantation is necessary for symmetric results as well as the ability to reduce the intensity and duration of off-periods as well increase the duration of on-periods.[1][4] The most effective structures used for implantations for deep brain stimulation are the internal globus pallidus (GPi) and the subthalamic nucleus (STN). This is because it is safer and more effective to alter the influence of the basal ganglia on the thalamocortical nuclei than directly altering neural activity in upper motor neuron circuits.[1] Deep brain stimulation is a more complicated process than other therapies such as ablation. Evidence suggests that benefits of STN deep brain stimulation is due to the activation of efferents and the modulation of discharge patterns in the GPi that are propagated throughout the thalamocortical pathways.[4] The ability to adjust stimulation protocols lends this treatment to a variety of disorders due its ability to alter the activity of basal ganglia circuits.[1]
See also
References
- ^ a b c d e f g h i Purves, D.; Augustine, G.; Fitzpatrick, D.; Hall, W.; LaManita, A.-S.; McNamara, J.; et al. (2008). Neuroscience (4th ed.). Sunderland MA: Sinauer Associates.
- ^ S2CID 30435859.
- S2CID 25483839.
- ^ PMID 17210805.
- ^ S2CID 24956799.
- ISBN 978-0-12-374767-9. Retrieved 20 April 2012.
- S2CID 46151626.
- PMID 9714810.
- ^ S2CID 422281.
- PMID 9771763.
- PMID 18669484.
- PMID 33381016.
- ^ "Sydenham Chorea Information Page" Archived 2010-07-22 at the Wayback Machine Saint Vitus Dance, Rheumatic Encephalitis from the National Institute of Neurological Disorders and Stroke. Accessed April 26, 2008
- ^ a b Sydenham's Chorea Symptoms.Accessed September 24, 2009. Archived April 18, 2008, at the Wayback Machine
- PMID 8464654.
- S2CID 23605799.
- PMID 30996598.
- S2CID 40827012.
- PMID 18495013.
- S2CID 30969859.
- PMID 22275846.
- ^ Hou, M; Zhao, J; Yu, R (2006). "Recent advances in dyskinetic cerebral palsy" (PDF). World J Pediatr. 2 (1): 23–28. Archived from the original (PDF) on 2016-03-04. Retrieved 2020-01-29.
- ^ "Athetoid Dyskinetic". Swope, Rodante P.A. Retrieved 31 October 2012.
- ^ a b Hou, M; Zhao, J; Yu, R (2006). "Recent advances in dyskinetic cerebral palsy" (PDF). World J Pediatr. 2 (1): 23–28. Archived from the original (PDF) on 2016-03-04. Retrieved 2020-01-29.
- ISBN 9780521774826.
- ^ Lesch–Nyhan syndrome. Genetics Home Reference. Retrieved on 2007-05-24.
- ISBN 0-7817-3473-8. [1]
- S2CID 2989668.
- S2CID 24663871.
- ^ "Chavany-Brunhes syndrome". Archived from the original on 2009-05-11. Retrieved 2009-06-13.
- PMID 17179586.
- ^ "Benign Essential Blepharospasm". NORD (National Organization for Rare Disorders). Retrieved 2020-01-29.
- S2CID 10245634.
