Bladder cancer
| Bladder cancer | |
|---|---|
Five-year survival rates ~77% (US)[2] | |
| Frequency | 549,000 new cases (2018)[3] |
| Deaths | 200,000 (2018)[3] |
Bladder cancer is any of several types of
Risk factors for bladder cancer include
Treatment depends on the
Bladder cancer, as of 2018, affected about 1.6 million people globally with 549,000 new cases and 200,000 deaths.[3] Age of onset is most often between 65 and 84 years of age.[2] Males are more often affected than females.[2] In 2018, the highest rate of bladder cancer occurred in Southern and Western Europe followed by North America with rates of 15, 13, and 12 cases per 100,000 people.[3] The highest rates of bladder cancer deaths were seen in Northern Africa and Western Asia followed by Southern Europe.[3]
Signs and symptoms
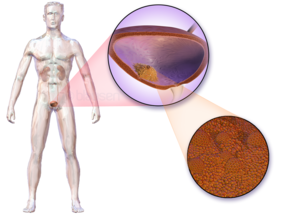
Bladder cancer characteristically causes
Other possible symptoms include
People with advanced disease may have pelvic or bony pain, lower-extremity swelling, or flank pain.[12] Rarely, a palpable mass can be detected on physical examination.[13]
Causes
Smoking
Tobacco smoking is the main known contributor to urinary bladder cancer; in most populations, smoking is associated with over half of bladder cancer cases in men and one-third of cases among women,[14] however these proportions have reduced over recent years since there are fewer smokers in Europe and North America.[15] There is an almost linear relationship between smoking duration (in years), pack years and bladder cancer risk. A risk plateau at smoking about 15 cigarettes a day can be observed (meaning that those who smoke 15 cigarettes a day are approximately at the same risk as those smoking 30 cigarettes a day). Smoking in any form (cigar, cigarette, pipe, Egyptian waterpipe and smokeless tobacco) increases the risk for bladder cancer.[16] Quitting smoking reduces the risk. Risk of bladder cancer decreases by 30% within 1–4 years and continues to decrease by 60% at 25 years after smoking cessation.[17] However, former smokers will most likely always be at a higher risk of bladder cancer compared to people who have never smoked.[15] Passive smoking also appears to be a risk.[18][19]
Opium consumption increases the risk of bladder cancer threefold, while concurrent use of opium and tobacco increases the risk of bladder cancer fivefold when compared to the general population.[20]
Occupational exposure
Thirty percent of bladder tumors probably result from occupational exposure in the workplace to carcinogens. Occupational or circumstantial exposure to the following substances has been implicated as a cause of bladder cancer;
Infection
Infection with
Diet
The American Institute for Cancer Research have stated that there is strong evidence that drinking water containing arsenic increases the risk of bladder cancer.[36]
High consumption of animal fat and dietary cholesterol increases bladder cancer risk in men.[37]
Ingestion of
Other
People who undergo external beam radiotherapy (EBRT) for prostate cancer have a higher risk of developing invasive bladder cancer.[39]
In addition to these major risk factors there are also numerous other modifiable factors that are less strongly (i.e. 10–20% risk increase) associated with bladder cancer, for example, obesity.[40] Although these could be considered as minor effects, risk reduction in the general population could still be achieved by reducing the prevalence of a number of smaller risk factor together.[41]
Genetics
Mutations in
Muscle invasive bladder cancer are heterogeneous in nature. In general, they can be genetically classified into basal and luminal subtypes. Basal subtype show alterations involving RB and NFE2L2 and luminal type show changes in FGFR3 and KDM6A genes.[51] Basal subtype are subdivided into basal and claudin low-type group and are aggressive and show metastasis at presentation, however they respond to platinum based chemotherapy. Luminal subtype can be subdivided into p53-like and luminal. p53-like tumors of luminal subtype although not as aggressive as basal type, show resistance to chemotherapy[52]
Diagnosis


Currently, the best diagnosis of the state of the bladder is by way of cystoscopy, which is a procedure in which a flexible or rigid tube (called a cystoscope) bearing a camera and various instruments is inserted into the bladder through the urethra. The flexible procedure allows for a visual inspection of the bladder, for minor remedial work to be undertaken and for samples of suspicious lesions to be taken for a biopsy. A rigid cystoscope is used under general anesthesia in the operating room and can support remedial work and biopsies as well as more extensive tumor removal. Unlike papillary lesion, which grow into the bladder cavity and are readily visible, carcinoma in situ lesion are flat and obscure. Detection of carcinoma in situ lesions requires multiple biopsies from different areas of interior bladder wall.[53] Photodynamic detection (blue light cystoscopy) can aid in the detection of carcinoma in situ. In photodynamic detection, a dye is instilled into the bladder with the help of a catheter. Cancer cells take up this dye and are visible under blue light, providing visual clues on areas to be biopsied or resected.[54]
However, visual detection in any form listed above, is not sufficient for establishing pathological classification, cell type or the stage of the present tumor. A so-called cold cup
If invasive or high grade (includes carcinoma in situ) cancer is detected on TURBT, an MRI and/or CT scan of the abdomen and pelvis or urogram and CT chest should be conducted for disease staging and to look for cancer spread (metastasis).[56] Increase in alkaline phosphatase levels without evidence of liver disease should be evaluated for bone metastasis by a bone scan.[57] Although 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT has been explored as a viable method for staging, there is no consensus to support its role in routine clinical evaluations.[54]
Classification

| Type | Relative incidence | Subtypes |
|---|---|---|
| Transitional cell carcinoma | 95%[66][67] | Papillary (70%[66]) |
| Non-papillary (30%[66]) | ||
| Non-transitional cell carcinoma | 5% [66][67] | small cell carcinomas, and secondary deposits from cancers elsewhere in the body.[67]
|
Non-papillary carcinoma includes carcinoma in situ (CIS), microinvasive carcinoma and frankly invasive carcinoma.[68] Carcinoma in situ (CIS) invariably consists of cytologically high-grade tumour cells.[69]
Transitional cell carcinoma can undergo differentiation (25%) into its variants.[68][70][71] When seen under a microscope, papillary transitional cell carcinoma can present in its typical form or as one of its variations (squamous, glandular differentiation or micropapillary variant). Different variations of non-papillary transitional cell carcinoma are listed below.
| Variant | Histology | Percentage of non-papillary cases | Implications[72] |
|---|---|---|---|
| Squamous differentiation | Presence of intercellular bridges or keratinization | 60% | Outcomes similar to conventional transitional cell carcinoma |
| Glandular differentiation | Presence of true glandular spaces | 10% | |
| Sarcomatoid foci | Presence of both epithelial and mesenchymal differentiation | 7% | Clinically aggressive[73] |
| Micropapillary variant | Resembles papillary serous carcinoma of the ovary or resembling micropapillary carcinoma of breast or lung[74] | 3.7% | Clinically aggressive, early cystectomy recommended |
| Urothelial carcinoma with small tubules and microcystic form | Presence of cysts with a size range of microscopic to 1-2mm | Rare | |
| Lymphoepithelioma-like carcinoma | Resembles lymphoepithelioma of the nasopharynx | ||
| Lymphoma-like and plasmacytoid variants | Malignant cells resemble cells of malignant lymphoma or plasmacytoma | ||
| Nested variant | Histologically look similar to von Brunn's nests | Can be misdiagnosed as benign von Brunn's nests or non-invasive low-grade papillary urothelial carcinoma | |
| Urothelial carcinoma with giant cells | Presence of epithelial tumour giant cells and looks similar to giant cell carcinoma of the lung | ||
| Trophoblastic differentiation | Presence of syncytiotrophoblastic giant cells or choriocarcinomatous differentiation, may express HCG | ||
| Clear cell variant | Clear cell pattern with glycogen-rich cytoplasm | ||
| Plasmacytoid | Cells with abundant lipid content, mimic signet ring cell adenocarcinoma of stomach/ lobular breast cancer | Clinically aggressive, propensity for peritoneal spread | |
| Unusual stromal reactions | Presence of following; pseudosarcomatous stroma, stromal osseous or cartilaginous metaplasia, osteoclast-type giant cells, lymphoid infiltrate |
Staging
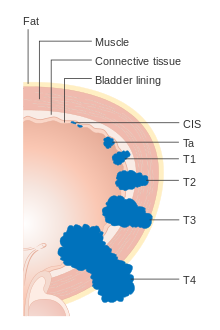




Bladder cancer is staged (classified by the extent of spread of the cancer) and graded (how abnormal and aggressive the cells appear under the microscope) to determine treatment and predict outcomes. Staging is usually performed with transurethral resection of bladder tumor (TURBT) and radiologic imaging (CT and MRI). Papillary tumors confined to the mucosa or which invade the lamina propria are classified as Ta or T1. Flat lesions that do not invade the basement membrane of the bladder mucosa are termed Tis (in situ). All three categories (Tis, Ta and T1) are grouped together as non-muscle invasive disease for therapeutic purposes and in most cases they are offered cystoscopic resection with TURBT without the need for radical resection of the entire urinary bladder. Tumors in the remaining categories (T2, T3 and T4) are termed muscle-invasive disease and are associated with less favorable prognosis.[56]
In the TNM staging system (8th Edn. 2017) for bladder cancer:[75][76]
T (Primary tumour)
- TX Primary tumour cannot be assessed
- T0 No evidence of primary tumour
- Ta Non-invasive papillary carcinoma
- Tis Carcinoma in situ ('flat tumour')
- T1 Tumour invades subepithelial connective tissue
- T2a Tumour invades superficial muscle (inner half of the detrusor muscle)[77]
- T2b Tumour invades deep muscle (outer half of the detrusor muscle)[77]
- T3 Tumour invades perivesical tissue:
- T3a Microscopically
- T3b Macroscopically (extravesical mass)
- T4a Tumour invades prostate, uterus or vagina
- T4b Tumour invades pelvic wall or abdominal wall
N (Lymph nodes)
- NX Regional lymph nodes cannot be assessed
- N0 No regional lymph node metastasis
- N1 Metastasis in a single lymph node in true pelvis (hypogastric, obturator, external iliac, or presacral nodes)
- N2 Metastasis in multiple lymph nodes in true pelvis (hypogastric, obturator, external iliac, or presacral nodes)
- N3 Metastasis in common iliac lymph nodes
M (Distant metastasis)
- MX Distant metastasis cannot be assessed
- M0 No distant metastasis
- M1 Distant metastasis.
- M1a: The cancer has spread only to lymph nodes outside of the pelvis.
- M1b: The cancer has spread other parts of the body.
The most common sites for bladder cancer metastases are the lymph nodes, bones, lung, liver, and
Numerical
The stages above can be integrated into a numerical staging (with Roman numerals) as follows:[81]
| Stage | Tumor | Nodes | Metastasis | 5-year survival in the US[82] |
|---|---|---|---|---|
| Stage 0a | Ta | N0 | M0 | 98% |
| Stage 0is | Tis | N0 | M0 | 95% |
| Stage I | T1 | N0 | M0 | 63% |
| Stage II | T2a | N0 | M0 | |
| T2b | ||||
| Stage IIIA | T3a | N0 | M0 | 35% |
| T3b | ||||
| T4a | ||||
| T1-4a | N1 | |||
| Stage IIIB | T1-4a | N2 | M0 | |
| N3 | ||||
| Stage IVA | T4b | Any N | M0 | |
| Any T | M1a | |||
| Stage IVB | Any T | Any N | M1b | 5% |
Grading
According to WHO classification (1973) bladder cancers are histologically graded into:[83]
- G1 – Well differentiated,
- G2 – Moderately differentiated
- G3 – Poorly differentiated
WHO classification (2004/2016)[84][85]
- Papillary lesions
- Urothelial Papilloma
- Papillary urothelial neoplasm of low malignant potential (PUNLMP)
- Low Grade
- High Grade
- Flat lesions
- Urothelial proliferation of uncertain malignant potential
- Reactive atypia
- Atypia of unknown significance
- Urothelial dysplasia
- Urothelial CIS (always high grade)
- Primary
- Secondary
- Concurrent
Risk stratification
People with non-muscle invasive bladder cancer (NMIBC), are risk-stratified based on clinical and pathological factors so that they are treated appropriately depending on their probability of having progression and/or recurrence.[86] People with non-muscle invasive tumors are categorized into low-risk, intermediate-risk and high-risk or provided with a numerical risk score. Risk-stratification framework is provided by American Urology Association/Society of Urological Oncology (AUA/SUO stratification), European Association of Urology (EAU) guidelines, European Organization for Research and Treatment of Cancer (EORTC) risk tables and Club Urológico Español de Tratamiento Oncológico (CUETO) scoring model.[87][88][89]
| Low risk | Intermediate risk | High risk |
|---|---|---|
| Low grade solitary Ta tumor, smaller than 3 cm | Recurrence within 1 year, Low grade Ta tumor | High grade T1 |
| Papillary urothelial neoplasm of low malignant potential | Solitary low grade Ta tumor, bigger than 3 cm | Any recurrent tumor or any high grade Ta |
| Low grade Ta, multifocal tumors | High grade Ta, bigger than 3 cm (or multifocal) | |
| High grade Ta, smaller than 3 cm | Any carcinoma in situ | |
| Low grade T1 | Any BCG failure in high grade tumors | |
| Any variant histology | ||
| Any lymphovascular invasion | ||
| Any high grade prostatic urethral involvement |
The EORTC and CUETO model use a cumulative score obtained from individual prognostic factors, which are then converted into risk of progression and recurrence. The six prognostic factors included in the EORTC model are number of tumors, recurrence rate, T-stage, presence of carcinoma-in-situ and grade of the tumor. Scoring for recurrence in the CUETO model incorporates 6 variables; age, gender, grade, tumor status, number of tumors and presence of tis. For progression scoring the previous 6 variables plus T stage is used.[90][91]
| Model | Cumulative score for recurrence | Recurrence at 1-year (%) | Recurrence at 5-year (%) |
|---|---|---|---|
| EORTC | 0 | 15 | 31 |
| 1-4 | 24 | 46 | |
| 5-9 | 38 | 62 | |
| 10-17 | 61 | 78 | |
| CUETO | 0-4 | 8.2 | 21 |
| 5-6 | 12 | 36 | |
| 7-9 | 25 | 48 | |
| 10-16 | 42 | 68 |
| Model | Cumulative score for progression | Progression at 1-year (%) | Progression at 5-year (%) |
|---|---|---|---|
| EORTC | 0 | 0.2 | 0.8 |
| 2-6 | 1 | 6 | |
| 7-13 | 5 | 17 | |
| 12-23 | 17 | 45 | |
| CUETO | 0-4 | 1.2 | 3.7 |
| 5-6 | 3 | 12 | |
| 7-9 | 5.5 | 21 | |
| 10-16 | 14 | 34 |
Prevention
Diet
As of 2019, there is limited high level evidence to suggest that eating vegetable and fruits decreases the risk of bladder cancer.
While it is suggested that the polyphenol compounds in tea may have an inhibitory effect on bladder tumor formation and growth, there is limited evidence to suggesting drinking tea decreases bladder cancer risk.[49] A 2022 review found that a Mediterranean diet has a protective effect on bladder cancer risk.[94] Higher consumption of monounsaturated fat and plant-based oils decrease bladder cancer risk in women.[37] Higher consumption of total dietary fiber and whole grains is associated with a reduced risk of bladder cancer.[95]
Screening
As of 2019 there is insufficient evidence to determine if screening for bladder cancer in people without symptoms is effective or not.[96]
Treatment

The treatment of bladder cancer depends on how deeply the tumor invades into the bladder wall.[citation needed]
Treatment strategies for bladder cancer include:[97][98]
- Non-muscle invasive: transurethral resection of bladder tumor (TURBT) with or without intravesical chemotherapy or immunotherapy
- Muscle invasive
- Stage II/Stage IIIA: radical cystectomy plus neoadjuvant chemotherapy (multimodal therapy, preferred) or transurethral resection with chemoradiation (trimodal therapy, highly selected people) or partial cystectomy plus neoadjuvant chemotherapy (in highly selected people)
- Stage IIIB/IVA: cisplatin-based chemotherapy followed by radical cystectomy or chemoradiation or observation depending on treatment response
- Stage IVB (locally advanced; unresectable tumors): palliative radiotherapy
- Metastatic disease: cisplatin-based chemotherapy
- Metastatic disease but unfit for cisplatin-based chemotherapy: carboplatin-based chemotherapy
- Metastatic disease with contraindication for chemotherapy: checkpoint inhibitors if programmed death ligand 1 (PD L1) positive
- Squamous cell carcinoma or adenocarcinoma of bladder: radical cystectomy
Non-muscle invasive
Transurethral resection
Non-muscle invasive bladder cancer (those not entering the muscle layer of the bladder) can be "shaved off" using an
Another method for reducing recurrence of tumors is medication after TURBT surgery. The two most common medicines used for this purpose are
Chemotherapy

A single instillation of chemotherapy into the bladder after primary TURBT has shown benefit in deceasing recurrence by 35% in non-muscle invasive disease.
Immunotherapy
BCG is delivered as induction and a maintenance course. The induction course consists of 6-week course of intravesical and percutaneous BCG.[114] This is followed by a maintenance course. There is no consensus regarding the maintenance schedule, however the most commonly followed is the Southwestern Oncology Group (SWOG) schedule.[115] The SWOG maintenance schedule consists of intravesical and percutaneous BCG every week for 3 weeks given at 3, 6, 12, 18, 24, 30 and 36 months.[114] Three weekly maintenance regimen with induction has shown complete response rate of 84% compared to 69% in people who received 6-week induction BCG only at 6 months. Many studies have explored alternate treatment schedules and regimes of BCG but has shown no clinical significance.[114] Efficacy of different strains of BCG (Connaught, TICE, Pasteur, Tokio-172) has been shown not to be different however, there is no high-level evidence.[116]
Side effects of BCG therapy include
Local infection (i.e.
BCG treatment failure
BCG treatment failure can be classified into 3 groups; BCG relapse, BCG-refractory and BCG-intolerant. In BCG relapse, tumor reoccurs after a disease free period. BCG-refractory tumors are the ones which do not respond to induction and maintenance doses of BCG or which progress during therapy. In BCG-intolerant, tumor reoccurs due to incomplete treatment as the person receiving it is unable to tolerate an induction course of BCG. Around 50% of the people fail BCG treatment and would require further treatment.[114]
People whose tumors recurred after treatment with BCG or who were unresponsive to treatment, are more difficult to treat.[120] In such people a radical cystectomy is recommendation[121][122] In people who do not show response to BCG therapy and are unfit or unwilling to undergo radical cystectomy, salvage therapies can be considered. Salvage therapy include intravesical chemotherapy with agents such as valrubicin, gemcitabine or docetaxel, chemoradiation or chemohyperthermia.[123]
| Risk | Other considerations | Chemotherapy | Immunotherapy (BCG) | Cystoscopy (surveillance) | Imaging (surveillance) |
|---|---|---|---|---|---|
| Low | at 3-months followed by cystoscopy at 12-months, then yearly for 5-years | CT/MR urography and CT/MRI of abdomen and pelvis at baseline | |||
| Intermediate | Primary tumor with history of chemotherapy | Intravesical chemotherapy for 1 year OR Intravesical BCG for 1 year (preferred) | at 3-months with cytology followed by once every 3–6 months for 5-years and then yearly | CT/MR urography and CT/MRI of abdomen and pelvis at baseline | |
| Recurrent tumors with history of previous chemotherapy | Intravesical BCG for 1 year | ||||
| High | Intravesical BCG for 3 year (as tolerated) | at 3-months with cytology followed by once every 3-months for 2-years after that, 6 monthly for 5 years then yearly | CT/MR urography and CT/MRI of abdomen and pelvis at baseline, CT/MR urography 1-2 yearly for 10 years | ||
| T1G3/High grade, Lymphovascular invasion, Presence of variant histology | Consider radical cystectomy | ||||
Muscle invasive

Multimodal therapy (standard treatment)
Untreated, non-muscle invasive tumors may gradually begin to infiltrate the muscular wall of the bladder (muscle invasive bladder cancer). Tumors that infiltrate the bladder wall require more radical surgery, where part (
A bilateral pelvic lymphadenectomy should accompany radical cystectomy. At minimum, a standard template of lymphadenectomy should be followed by removing the external and internal iliac and obturator lymph node.[124] When performing a lymphadenectomy, the surgeon can either remove lymph nodes from a smaller (standard) or from larger (extended) area. In comparison with a standard lymph node dissection, having an extended dissection may reduce a person's likelihood of death from any reason, including dying from bladder cancer.[125] The extended procedure may lead to more serious unwanted effects and may or may not influence the likelihood of the cancer recurring over time.[125] The rate of not-so-serious side effects may be similar for both surgeries.[125]
Radical cystectomy has a significant morbidity associated with it. About 50-65% of the people experience complication within 90 days of surgery.[126][127] Mortality rates was 7% within 90 days of surgery. High volume centers have better outcomes than low volume centers.[128] Some centers are implementing Enhanced Recovery After Surgery (ERAS) society recommendations to decrease morbidity after radical cystectomy. However, due to lack of specific evidence in urologic surgery, ERAS protocol has not been widely incorporated in urologic clinical care.[129]
Even after surgical removal of bladder, 50% of the people with muscle invasive disease (T2-T4) develop metastatic disease within two years due to micrometastasis.[130] In such, neoadjuvant chemotherapy (chemotherapy before main treatment, i.e. surgery) has shown to increase overall survival at 5 years from 45% to 50% with an absolute survival benefit of 5%.[131][132][133] Currently the two most used chemotherapy regimens for neoadjuvant chemotherapy are platinum based; methotrexate, vinblastine, doxorubicin, cisplatin (MVAC) and gemcitabine with cisplatin (GC).[134] Other regimens include dose dense MVAC (DDMVC) and cisplatin, methotrexate and vinblastine (CMV). Although, the optimal regimen has not been established, the preferred regimen for neoadjuvant therapy is MVAC.[134]
Role of adjuvant chemotherapy (chemotherapy after main treatment) is limited to people with high grade tumours (pT3/T4and/or N+) and who have not been treated with neoadjuvant therapy.[124] Adjuvant radiation therapy has not shown any advantage in bladder cancer treatment.[135]
| MVAC | DDMVAC | Gemcitabine + cisplatin |
|---|---|---|
| Methotrexate (30 mg/m2 IV) - day 1,15,22
Vinblastine (3 mg/m2 IV) - day 2, 15, 22 Doxorubicin (30 mg/m2 IV) - day 2 Cisplatin (70 mg/m2 IV) - day 2 Repeat every 4 weeks for 3 cycles |
Methotrexate (30 mg/m2 IV) - day 1 Vinblastine (3 mg/m2 IV) - day 2 Doxorubicin (30 mg/m2 IV) - day 2 Cisplatin (70 mg/m2 IV) - day 2 Granulocyte colony-stimulating factor (G-CSF) (240μg/m2 SC) - day 4-10 Repeat every 2 weeks for 3–4 cycles |
Gemcitabine (1,000 mg/m2 IV) - day 1,8,15 Cisplatin (70 mg/m2) - day 2 Repeat every 4 weeks for 4 cycles |
Trimodal therapy (alternative treatment)
A combination of radiation and chemotherapy (chemoradiation) in conjunction with transurethral (endoscopic) bladder tumor resection can be used as an alternative in certain people.[138] Review of available large data series on this so-called trimodality therapy has indicated similar long-term cancer specific survival rates, with improved overall quality of life as for people undergoing radical cystectomy with urinary reconstruction. However, currently no randomized control trials are available which has compared trimodal therapy with radical cystectomy. People who undergo trimodal therapy are usually highly selected and generally have T2 disease without hydronephrosis and/or carcinoma in-situ.[139] Five year cancer specific survival and overall survival after trimodal therapy is between 50% to 82% and 36% to 74%.[138]
In trimodal therapy, a maximal TURBT is conducted followed by chemoradiation therapy. Radiation sensitizing chemotherapy regimens consisting of cisplatin or 5-flurouracil and mitomycin C are used.
In people who fail trimodal therapy, radical cystectomy is considered if there is muscle invasive or recurrent tumors. Around 25-30% fail treatment and undergo salvage radical cystectomy.[138] TURBT with intravesical therapy is indicated after treatment failure for non-muscle invasive disease.[124]
Partial cystectomy
In people with solitary tumor without concurrent carcinoma in situ in an area where a clean surgical margins can be achieved, a partial cystectomy with lymphadenectomy can be considered. Management plan including partial cystectomy should be accompanied with neoadjuvant chemotherapy.[98] In people with urachal adenocarcinoma of the bladder, a partial cystectomy with en-bloc resection of urachal ligament and umbilicus can be considered.[11]
Metastatic disease
First line treatment
Cisplatin-containing combination chemotherapy is the standard of care for metastatic bladder care.[142] Fitness for receiving cisplatin based chemotherapy is assessed before treatment. A person is deemed unfit if anyone of the following is true.[143]
- Eastern Cooperative Oncology Group performance status of 2
- Creatinine clearance< 60 mL/min
- Grade ≥ 2 hearing loss
- Grade ≥ 2 neuropathy
- New York Heart Association Class III heart failure
People who are deemed fit receive platinum based regimens;
People with bone metastasis should receive
| DDMVAC | Gemcitabine + Cisplatin |
|---|---|
| Methotrexate (30 mg/m2 IV) - day 1
Vinblastine (3 mg/m2 IV) - day 2 Doxorubicin (30 mg/m2 IV) - day 2 Cisplatin (70 mg/m2 IV) - day 2 Granulocyte colony-stimulating factor (G-CSF) (240μg/m2 SC) - day 4-10 Repeat every 2 weeks for 3–4 cycles |
Gemcitabine (1,000 mg/m2 IV) - day 1,8,15 Cisplatin (70 mg/m2) - day 2 Repeat every 4 weeks for 4 cycles |
| Atezolizumab (in PD-L1+) | Gemcitabine + Carboplatin | Pembrolizumab (in PD-L1+) |
|---|---|---|
| Atezolizumab (Atezolizumab 1200 mg IV)
every 3 weeks |
Gemcitabine (1,000 mg/m2 IV) - day 1,8 Carboplatin (4.5 × [glomerular filtration rate + 25]) - day 1 and every 3 weeks |
Pembrolizumab 200 mg every 3 weeks |
Second line treatment
Bladder cancer that is refractory or shows progression after platinum-based chemotherapy can be treated with second-line chemotherapy or immunotherapy.[citation needed]
The most commonly used second-line chemotherapy is single-agent regimes of
In people with fibroblast growth factor receptors (FGFR) mutations and fail standard platinum based chemotherapy erdafitinib can be used. Erdafitinib has shown a response rate of 40% in these patients.[154]
Five immunotherapy agents have been approved in the US for use in metastatic bladder cancer. They act by inhibiting programmed cell-death protein 1 (PD-1) or programmed cell-death ligand 1 (PD-L1). Pembrolizumab and nivolumab, and are inhibitors of programmed cell-death ligand 1 (PD-1). Avelumab, atezolizumab and durvalumab are inhibitors of PD-L1.[155][156]
Pembrolizumab probably improves overall survival a little and may slightly improve quality of life for people with
| Atezolizumab | Nivolumab | Pembrolizumab | Durvalumab | Avelumab |
|---|---|---|---|---|
| Atezolizumab 1200 mg IV
every 3 weeks |
Nivolumab 3 mg/kg IV every 2 weeks |
Pembrolizumab 200 mg every 3 weeks |
Durvalumab 10 mg/kg every 2 weeks for 12 months |
Avelumab 10 mg/kg IV every 2 weeks |
Surveillance and response
Contrast enhanced
Prognosis
People with non-muscle invasive tumors have a favorable outcome (5-year survival is 95% vs. 69% of muscle invasive bladder cancer).[160][161] However, 70% of them will have a recurrence after initial treatment with 30% of them presenting with muscle invasive disease.[162] Recurrence and progression to a higher disease stage have a less favorable outcome.[163]
Survival after
There are several prognostic factors which determine cancer specific survival after radical cystectomy. Factor with detrimental effect of cancer specific survival are old age, higher tumor grade and pathological stage, lymph node metastasis, presence of lymphovascular invasion and positive soft tissue margin.[165] Lymph node density (positive lymph nodes/total lymph nodes observed in the specimen from surgery) is a predictor of survival in lymph node positive disease. Higher the density lower is the survival.[166]
Quality of life
After radical cystectomy, urinary and sexual function remain inferior to the general population. People who have a neobladder have better emotional function and body image compared with ones with cutaneous diversion (who need to wear a bag to collect urine over their abdomen).[167] Social factors such as family, relationships, health and finances contribute significantly for determining good quality of life in people who have been diagnosed with bladder cancer.[168]
A high percentage of people with bladder cancer have anxiety and depression.[169] People who are young, single and have advanced clinical disease have a high risk for getting diagnosed with a psychiatric illness post-treatment. People with psychiatric illness post treatment seem to have worse cancer specific and overall survival.[170][171]
Epidemiology
| Rank | Country | Overall | Men | Women |
|---|---|---|---|---|
| 1 | Lebanon | 25 | 40 | 9 |
| 2 | Greece | 21 | 40 | 4 |
| 3 | Denmark | 18 | 29 | 8 |
| 4 | Hungary | 17 | 27 | 9 |
| 5 | Albania | 16 | 27 | 6 |
| 5 | Netherlands | 16 | 26 | 8 |
| 7 | Belgium | 16 | 27 | 6 |
| 8 | Italy | 15 | 27 | 6 |
| 9 | Germany | 15 | 26 | 6 |
| 10 | Spain | 15 | 27 | 6 |
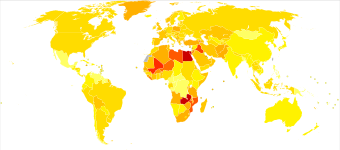
Globally, in 2017, bladder cancer resulted in 196,000 deaths, a 5.4% (
The risk of bladder cancer occurrence is four times higher in men than in women.[3] Smoking can only partially explain this higher rates in men in western hemisphere.[177] One other reason is that the androgen receptor, which is much more active in men than in women, may play a part in the development of the cancer.[178] This hypothesis is also supported by the fact that men undergoing androgen suppression therapy for unrelated reason seem to have a lower risk of developing bladder cancer.[179] In Africa, men are more prone to do field work and are exposed to infection with Schistosoma, this may explain to a certain extent the gap in incidence of squamous cell cancers in areas where bladder cancer is endemic.[177] However, women present with more aggressive disease and have worse outcomes than men. This difference in outcomes is attributed to numerous factors such as, difference in carcinogen exposure, genetics, social and quality of care.[50] One of the common signs of bladder cancer is hematuria and is quite often misdiagnosed as urinary tract infection in women, leading to a delay in diagnosis.[50] Moreover, as mentioned earlier PSCA gene may play a role in aggressive tumors in women.[citation needed]
Canada
Bladder cancer is the sixth most common cancer accounting for 3.7% of the new cancer cases. in 2018, 30,700 Canadians were living with bladder cancer, 9160 new cases were diagnosed and 2467 died from it.[180] In 2019, it is estimated that 11,800 new cases will be diagnosed and 2500 will die from it.[181] Among the 11,800 new cases, 9100 will be in men and 2700 in women. Of the 2500 who would die from it, 1800 will be men and 700 will be women.[181]
China
Bladder cancer is the 14th most common cancer and 16th most common cause of cancer death. In, 2018 it accounted for 82,300 new cases and 38,200 deaths.[182] The number of new cases is comparatively lower compared to its western counterparts. Majority of the people are diagnosed with non-muscle invasive disease (75%) and the rest have muscle invasive disease (25%). Carcinoma in situ was present in only 2.4% of the cases.[183]
Europe
In 2015, 131,000 news cases were diagnosed in the European Union with 40,000 deaths. It is the 5th most common cancer and 9th most common cause of cancer deaths.. The 5-year relative survival for bladder cancers diagnosed between 2000 and 2007 is 69%. Geographic variation is seen in survival rates with 5-year survival rates of 75% in Northern to 65% in Eastern Europe.[184]
United Kingdom
Bladder cancer is the ninth most common cancer in the
United States
In the United States in 2019 80,470 cases and 17,670 deaths are expected making it the sixth most common type of cancer in the region.[2] Bladder cancer is the fourth most common type of cancer in men and the 12th most common cancer in women.[186] Around 62,000 men and 19,000 women are diagnosed with bladder cancer in 2019.[187] Between 2012 and 2016 annual rate of new bladder cancer cases decreased by one percent per year.[188]
Veterinary medicine
References
- ^ a b c d e f g h i j k l m n "Bladder Cancer Treatment (PDQ®)–Patient Version - National Cancer Institute". www.cancer.gov. 11 May 2020. Retrieved 4 June 2020.
- ^ a b c d e f "Cancer of the Urinary Bladder - Cancer Stat Facts". SEER. Retrieved 30 October 2019.
- ^ a b c d e f "Bladder Cancer Factsheet" (PDF). Global Cancer Observatory. Retrieved 8 November 2019.
- ^ Heyes S.M., Prior K.N., Whitehead D., Bond M.J. Toward an Understanding of Patients' and Their Partners' Experiences of Bladder Cancer. Cancer Nurs.. 2020;43(5):E254-E263. doi:10.1097/NCC.0000000000000718
- ^ "Bladder Cancer Treatment". National Cancer Institute. 5 June 2017. Archived from the original on 14 July 2017. Retrieved 18 July 2017.
- ^ "EAU Guidelines: Non-muscle-invasive Bladder Cancer". Uroweb.
- ^ "Bladder Cancer - Stages and Grades". Cancer.Net. 25 June 2012.
- ^ "Bladder cancer". World Cancer Research Fund. 24 April 2018.
- ^ "Survival statistics for bladder cancer - Canadian Cancer Society". www.cancer.ca.
- S2CID 41691491.
- ^ PMID 27785426.
- OCLC 1089396489.)
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link - PMID 29094888.
- PMID 10931463.
- ^ PMID 27097748.
- PMID 22759493.
- PMID 10738259.
- PMID 30288109.
- ^ "Health Risks of Secondhand Smoke". www.cancer.org. Retrieved 21 November 2019.
- PMID 28586371.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risk to Humans (2012). 4-AMINOBIPHENYL. International Agency for Research on Cancer.
- PMID 24889821.
- PMID 25377503.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risk to Humans (2012). CHLORNAPHAZINE. International Agency for Research on Cancer.
- ^ Humans, IARC Working Group on the Evaluation of Carcinogenic Risk to (2012). 2-NAPHTHYLAMINE. International Agency for Research on Cancer.
- S2CID 30510231.
- PMID 20647380.
- PMID 20447989.
- PMID 9880476.
- PMID 23159285.
- PMID 8149471.
- PMID 8697435.
- PMID 9043502.
- S2CID 10487371.
- PMID 20039416.
- ^ "Diet, nutrition, physical activity and bladder cancer". wcrf.org. Retrieved 27 October 2022.
- ^ PMID 35182086.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - PMID 25431765.
- PMID 24223022.
- PMID 25803438.
- PMID 27000312.
- ^ "Cancer Genetics Browser". cancer.sanger.ac.uk. Retrieved 21 November 2019.
- ^ Online Mendelian Inheritance in Man (OMIM): 109800
- S2CID 14316154.
- ^ "Bladder cancer". Genetics Home Reference.
- PMID 22422829.
- S2CID 3756845.
- PMID 12117698.
- ^ a b c "Bladder Cancer Report" (PDF). World Cancer Research Fund : International. Retrieved 9 November 2019.
- ^ PMID 27785424.
- PMID 28365159.
- S2CID 24723395.
- ^ "EAU Guidelines: Non-muscle-invasive Bladder Cancer Diagnosis". Uroweb. Retrieved 12 November 2019.
- ^ a b "EAU Guidelines: Muscle-invasive and Metastatic Bladder Cancer diagnosis". Uroweb. Retrieved 12 November 2019.
- ^ a b c "Uroweb - European Association of Urology (EAU)". Uroweb. Retrieved 7 November 2019.
- ^ S2CID 249869037. Retrieved 23 June 2022.
- ^ "Bladder Cancer Treatment". National Cancer Institute. 8 May 2020. Retrieved 4 June 2020.
- PMID 12559279.
- PMID 18660854.
- ^ PMID 26501851.
- PMID 30245975.
- ^ PMID 30588457.
- ^ PMID 23479428.
- ^ Health, Center for Devices and Radiological (7 October 2019). "Nucleic Acid Based Tests". FDA.
- PMID 29931526.
- ^ PMID 27737647.
- ^ a b c "Types of Bladder Cancer: TCC & Other Variants". CancerCenter.com. Archived from the original on 10 August 2018. Retrieved 10 August 2018.
- ^ PMID 19494856.
- PMID 26622320.
- PMID 20019984.
- S2CID 6351401.
- PMID 28982751.
- PMID 24587922.
- ^ "Urothelial Carcinoma Variants - American Urological Association". www.auanet.org. Archived from the original on 30 July 2020. Retrieved 3 November 2019.
- ^ "EAU Guidelines - STAGING AND CLASSIFICATION SYSTEMS". Uroweb.
- PMID 30565300.
- ^ Cancer.net. 25 June 2012. Approved by the Cancer.Net Editorial Board 05/2019
- PMID 21178055.
- ^ .
- PMID 30456183.
- ^ "How is bladder cancer staged?". American Cancer Society. Archived from the original on 4 October 2015. Last Medical Review: 11/02/2019
- ^ "Survival rates for bladder cancer by stage". American Cancer Society. Archived from the original on 13 October 2015. Last Medical Review: 02/26/2014
- ^ Seth P. Lerner. "Overview of Diagnosis and Management of Non-Muscle Invasive Bladder Cancer" (PDF). Food and Drug Administration. ODAC 14 September 2016
- PMID 9850170.
- PMID 29366854.
- ^ PMID 27317986.
- ^ a b "Bladder Cancer: Non-Muscle Invasive Guideline - American Urological Association". www.auanet.org. Archived from the original on 7 August 2020. Retrieved 30 October 2019.
- S2CID 53722156.
- PMID 16442208.
- PMID 16442208.
- PMID 19758621.
- ^ "EAU Guidelines: Non-muscle-invasive Bladder Cancer". Uroweb.
- PMID 25324946.
- PMID 35016647.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - PMID 32778880.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - ^ "Final Update Summary: Bladder Cancer in Adults: Screening - US Preventive Services Task Force". www.uspreventiveservicestaskforce.org. Retrieved 13 November 2019.
- S2CID 29104789.
- ^ a b c "NCCN Bladder cancer guidelines 2018" (PDF). Retrieved 25 November 2019.
- PMID 25001887.
- PMID 25245244.
- S2CID 5784606.
- ^ a b "EAU Guidelines: Non-muscle-invasive Bladder Cancer". Uroweb.
- ^ PMID 31912907.
- ^ PMID 30976571.
- PMID 17719169.
- S2CID 24496739.
- PMID 27635313.
- S2CID 681090.
- S2CID 19355109.
- PMID 1922207.
- PMID 8423234.
- PMID 8102183.
- PMID 26000263.
- ^ PMID 25800393.
- PMID 30915163.
- PMID 30976572.
- PMID 25210559.
- PMID 26413275.
- PMID 26605208.
- S2CID 5103506.
- ^ Babjuk W, Oosterlinck W, Sylvester R, et al. (2010). "Guidelines on TaT1 (Non-muscle invasive) Bladder Cancer". European Association of Urology. Archived from the original on 24 April 2010.
- ^ Bladder Cancer Clinical Guideline Update Panel (2007). Bladder Cancer: Guideline for the Management of Nonmuscle Invasive Bladder Cancer: (Stages Ta, T1, and Tis): 2007 Update. American Urological Association.[page needed]
- S2CID 73439134.
- ^ a b c d "Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline (2017) - American Urological Association". www.auanet.org. Archived from the original on 23 July 2020. Retrieved 20 November 2019.
- ^ PMID 31111956.
- S2CID 7369962.
- PMID 18675501.
- PMID 28723446.
- S2CID 24108744.
- ^ "UpToDate: Neoadjuvant chemotherapy". www.uptodate.com.
- S2CID 29233494.
- PMID 15939524.
- PMID 12944571.
- ^ PMID 27053504.
- S2CID 195760627.
- ^ a b c d "Bladder Cancer Treatment Regimens". Cancer Therapy Advisor. 1 January 2019. Archived from the original on 29 August 2021. Retrieved 26 November 2019.
- ^ "UpToDate". www.uptodate.com. Retrieved 26 November 2019.
- ^ PMID 24613684.
- ^ Smelser WW, Austenfeld MA, Holzbeierlein JM, Lee EK. Where are we with bladder preservation for muscle-invasive bladder cancer in 2017?. Indian J Urol 2017;33:111–7 http://www.indianjurol.com/text.asp?2017/33/2/111/203415 Archived 10 September 2017 at the Wayback Machine
- PMID 27376137.
- ^ "Chemotherapy for Bladder Cancer". www.cancer.org. Retrieved 27 November 2019.
- ^ "EAU Guidelines: Muscle-invasive and Metastatic Bladder Cancer". Uroweb.
- S2CID 33263969.
- ^ ISBN 978-0-12-809939-1. Retrieved 27 November 2019.
- PMID 23788754.
- ^ Gilligan TD, Steele GS, Zietman AL, Kantoff PW (2003). Chemotherapy for Metastatic Disease. BC Decker.
- PMID 21543626.
- S2CID 26003400.
- PMID 17906299.
- PMID 28489981.
- PMID 28042310.
- PMID 26410730.
- ISBN 978-0-12-809939-1. Retrieved 27 November 2019.
- ^ "Erdafitinib Effective Against Advanced Bladder Cancer". National Cancer Institute. 9 August 2019.
- S2CID 73472390.
- S2CID 22280860.
- ^ PMID 30036453.
- PMID 20693823.
- ^ Llewelyn R. "Response evaluation criteria in solid tumors | Radiology Reference Article | Radiopaedia.org". Radiopaedia.
- PMID 28778250.
- ^ "Bladder Cancer - Statistics". Cancer.Net. 25 June 2012.
- S2CID 40417997.
- .
- PMID 28261632.
- PMID 31324162.
- PMID 26027955.
- PMID 27566035.
- PMID 19773042.
- PMID 30112443.
- ^ David E (February 2017). "Psychiatric disorders worsen bladder cancer survival". www.healio.com. Retrieved 27 November 2019.
- PMID 29660788.
- ^ "Bladder cancer statistics". World Cancer Research Fund. 22 August 2018. Retrieved 9 November 2019.
- ^ "Greece Factsheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 11 November 2009. Retrieved 11 November 2009.
- PMID 30496103.
- PMID 30027755.
- ^ S2CID 28518800.
- ^ "Scientists Find One Reason Why Bladder Cancer Hits More Men". University of Rochester Medical Center. 20 April 2007. Archived from the original on 11 January 2009. Retrieved 20 April 2007.
- S2CID 208017350.
- ^ "Canada Fact Sheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- ^ a b "Bladder cancer statistics - Canadian Cancer Society". www.cancer.ca. Retrieved 2 December 2019.
- ^ "China Fact Sheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- PMID 27049022.
- ^ team, FPFIS (8 March 2017). "Epidemiology of bladder cancer in Europe". EU Science Hub - European Commission. Retrieved 2 December 2019.
- ^ "UK fact sheet" (PDF). Global Cancer Observatory. Retrieved 9 November 2019.
- PMID 31145458.
- ^ "Key Statistics for Bladder Cancer". www.cancer.org. Retrieved 28 November 2019.
- ^ "Common Cancer Sites - Cancer Stat Facts". SEER. Retrieved 28 November 2019.
- ISBN 9780323263375.
External links
- Bladder cancer at Curlie
