Blood–brain barrier
| Blood–brain barrier | |
|---|---|
 Solute permeability at the BBB vs. choroid plexus | |
| Details | |
| System | Neuroimmune system |
| Identifiers | |
| Acronym(s) | BBB |
| MeSH | D001812 |
| Anatomical terminology | |
The blood–brain barrier (BBB) is a highly selective
The blood–brain barrier restricts the passage of
Specialized brain structures participating in sensory and secretory integration within brain
Structure

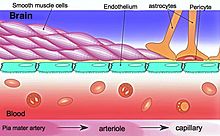
The BBB results from the selectivity of the
The BBB is composed of endothelial cells restricting passage of substances from the blood more selectively than endothelial cells of capillaries elsewhere in the body.
Not all vessels in the human brain exhibit BBB properties. Some examples of this include the circumventricular organs, the roof of the third and fourth ventricles, capillaries in the pineal gland on the roof of the diencephalon and the pineal gland. The pineal gland secretes the hormone melatonin "directly into the systemic circulation",[12] thus melatonin is not affected by the blood–brain barrier.[13]
Development
The BBB appears to be functional by the time of birth.
Measurement of brain uptake of various blood-borne solutes showed that newborn endothelial cells were functionally similar to those in adults,[15] indicating that a selective BBB is operative at birth.
In mice, Claudin-5 loss during development is lethal and results in size-selective loosening of the BBB.[16]
Function
The blood–brain barrier acts effectively to protect brain tissue from circulating
Circumventricular organs
Permeable capillaries of the sensory CVOs (area postrema, subfornical organ, vascular organ of the lamina terminalis) enable rapid detection of circulating signals in systemic blood, while those of the secretory CVOs (median eminence, pineal gland, pituitary lobes) facilitate transport of brain-derived signals into the circulating blood.
Specialized permeable zones
The border zones between brain tissue "behind" the blood–brain barrier and zones "open" to blood signals in certain CVOs contain specialized hybrid capillaries that are leakier than typical brain capillaries, but not as permeable as CVO capillaries. Such zones exist at the border of the area postrema—
Therapeutic research
As a drug target
The blood–brain barrier is formed by the brain capillary endothelium and excludes from the brain 100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs.[1] Overcoming the difficulty of delivering therapeutic agents to specific regions of the brain presents a major challenge to treatment of most brain disorders.[27][28] In its neuroprotective role, the blood–brain barrier functions to hinder the delivery of many potentially important diagnostic and therapeutic agents to the brain. Therapeutic molecules and antibodies that might otherwise be effective in diagnosis and therapy do not cross the BBB in adequate amounts to be clinically effective.[27] The BBB represents an obstacle to some drugs reaching the brain, thus to overcome this barrier some peptides able to naturally cross the BBB have been widely investigated as a drug delivery system.[29]
Mechanisms for drug targeting in the brain involve going either "through" or "behind" the BBB. Modalities for
Other methods used to get through the BBB may entail the use of endogenous transport systems, including carrier-mediated transporters, such as glucose and amino acid carriers, receptor-mediated
Nanoparticles
Damage in injury and disease
The blood–brain barrier may become damaged in select
Prediction
There have been many attempts to correlate the experimental blood–brain barrier permeability with
The first papers modelling blood-brain barrier permeability identified three properties, i.e., molecular volume, lipophilicity, and hydrogen bonding potential, as contributing to solute transport through the blood-brain barrier.[41] A 2022 dataset selected different classification models[42] based on molecular fingerprints,[43] MACCS166 keys[44] and molecular descriptors.[45]
History
A 1898 study observed that low-concentration "
Two years later, Max Lewandowsky may have been the first to coin the term "blood–brain barrier" in 1900, referring to the hypothesized semipermeable membrane.[47] There is some debate over the creation of the term blood–brain barrier as it is often attributed to Lewandowsky, but it does not appear in his papers. The creator of the term may have been Lina Stern.[48] Stern was a Russian scientist who published her work in Russian and French. Due to the language barrier between her publications and English-speaking scientists, this could have made her work a lesser-known origin of the term.
All the while,
However, in a later experiment in 1913, Edwin Goldmann (one of Ehrlich's students) injected the dye directly into the cerebrospinal fluid of animal brains. He found then the brains did become dyed, but the rest of the body did not, demonstrating the existence of a compartmentalization between the two. At that time, it was thought that the blood vessels themselves were responsible for the barrier, since no obvious membrane could be found.
See also
- blood–ocular barrier – Physical barrier between the local blood vessels and most parts of the eye itself
- blood–retinal barrier – Part of the blood–ocular barrier that prevents certain substances from entering the retina
- blood–saliva barrier – Semipermeable biological barrier
- blood–spinal cord barrier – Semipermeable anatomical interface
- blood–testis barrier – Physical barrier between the blood vessels and the seminiferous tubules of animal testes
References
- ^ PMID 25561720.
- S2CID 2202060.
- S2CID 91847478. Retrieved 2023-11-02.
- PMID 24309662.
- PMID 33208141.
- ^ PMID 19506719.
- PMID 23072749.
- PMID 28177105.
- PMID 29437557.
- S2CID 205500476.
- PMID 17922819.

- OCLC 41086829.
- S2CID 15395925.
- S2CID 46815691.
- S2CID 21944159.
- PMID 12743111.
- ^ PMID 29949404.
- PMID 15808097.
- PMID 21349155.
- PMID 23823990.
- ^ PMID 2891718.
- ^ PMID 1410407.
- ^ PMID 26578857.
- ^ S2CID 44760261.
- ^ PMID 2260724.
- ^ S2CID 27789146.
- ^ PMID 29377008.
- S2CID 215807970.
- PMID 35402305.
- PMID 27531677.
- PMID 18207311.
- PMID 23650374.
- PMID 23157552.
- ^ PMID 19091001.
- PMID 10751361.
- PMID 30546844.
- PMID 26973468.
- PMID 25477782.
- S2CID 17688028.
- PMID 2894467.
- S2CID 22184045.
- PMID 36361669.
- PMID 20426451.
- PMID 12444722.
- ISBN 978-3-527-31852-0.
- ^ Biedl A, Kraus R (1898). "Über eine bisher unbekannte toxische Wirkung der Gallensäuren auf das Zentralnervensystem" [A previously unknown toxic effect of bile acids on the central nervous system]. Zentralblatt Inn Med. 19: 1185–1200. Google Scholar: 4353654721035571173
- ^ a b "History of Blood-Brain Barrier". Davis Lab. The University of Arizona. Retrieved 2023-11-02.
- PMID 25565938.
- ^ PMID 26578854.
