Boyland–Sims oxidation
Source: Wikipedia, the free encyclopedia.
| Boyland–Sims oxidation | |
|---|---|
| Named after | Eric Boyland Peter Sims |
| Reaction type | Organic redox reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000181 |
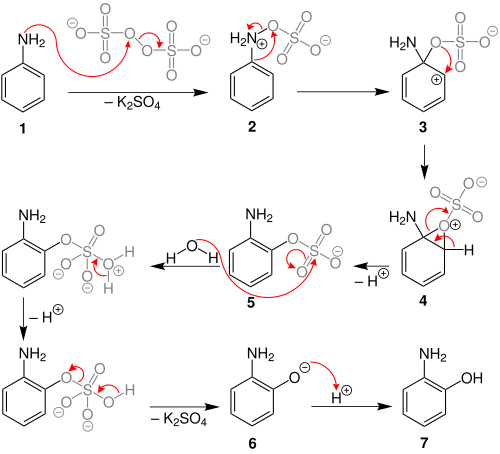
The Boyland–Sims oxidation is the chemical reaction of anilines with alkaline potassium persulfate, which after hydrolysis forms ortho-hydroxyl anilines.[1][2][3] The reaction is generally performed in water at room temperatures or below, using equimolar quantities of reagents.

The ortho-isomer is formed predominantly. However, the para-sulfate is formed in small amounts with certain anilines.[4]
Scope and mechanism
The reaction is disadvantaged by moderate to low
chemical yields, but is simple to perform and uses mild conditions. Some competitive oxidation of the nitrogen has been observed.[3]
Behrman has shown that the first intermediate in the Boyland–Sims oxidation is the formation of an arylhydroxylamine-O-sulfate (2).ortho- sulfate (5), which then hydrolyses to form the ortho-hydroxyl aniline.
See also
References
- .
- .
- ^ ISBN 0471264180.
- PMID 13315210.
- .
Further reading
- Behrman, Edward J. (2014). "On the Mechanism of the Boyland-Sims Oxidation". Progress in Reaction Kinetics and Mechanism. 39 (3). Science Reviews 2000 Ltd: 308–310. S2CID 101779652. Archived from the originalon 2016-07-16. Retrieved 2015-11-06.
