Branched-chain amino acid
This article needs more primary sources. (November 2018) |  |
A branched-chain amino acid (BCAA) is an


The three proteinogenic BCAAs are among the nine essential amino acids for humans, accounting for 35% of the essential amino acids in muscle proteins and 40% of the preformed amino acids required by mammals.[2] Synthesis for BCAAs occurs in all locations of plants, within the plastids of the cell, as determined by presence of mRNAs which encode for enzymes in the metabolic pathway.[3][4][5] Oxidation of BCAAs may increase fatty acid oxidation and play a role in obesity. Physiologically, BCAAs take on roles in the immune system and in brain function. BCAAs are broken down effectively by dehydrogenase and decarboxylase enzymes expressed by immune cells, and are required for lymphocyte growth and proliferation and cytotoxic T lymphocyte activity.[4] Lastly, BCAAs share the same transport protein into the brain with aromatic amino acids (Trp, Tyr, and Phe). Once in the brain BCAAs may have a role in protein synthesis, synthesis of neurotransmitters, and production of energy.[4]
Requirements
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For leucine, for adults 19 years and older, 42 mg/kg body weight/day; for isoleucine 19 mg/kg body weight/day; for valine 24 mg/kg body weight/day.[6] For a 70 kg (154 lb) person this equates to 2.9, 1.3 and 1.7 g/day. Diets that meet or exceed the RDA for total protein (0.8 g/kg/day; 56 grams for a 70 kg person), meet or exceed the RDAs for branched-chain amino acids.
Synthesis
Five enzymes participate in the parallel synthesis pathways for isoleucine, valine, and leucine: threonine dehydrogenase, acetohydroxyacid synthase, ketoacid reductoisomerase, dihydroxyacid dehydrogenase and
Degradation
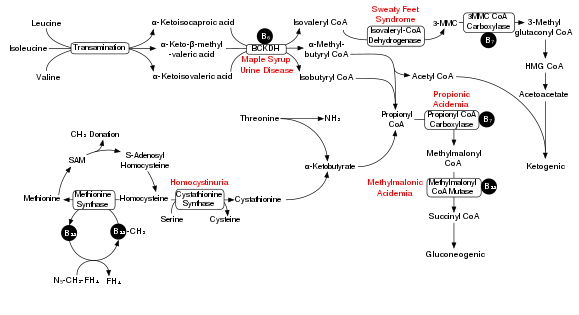
Degradation of branched-chain amino acids involves the branched-chain alpha-keto acid dehydrogenase complex (BCKDH). A deficiency of this complex leads to a buildup of the branched-chain amino acids (leucine, isoleucine, and valine) and their toxic by-products in the blood and urine, giving the condition the name maple syrup urine disease. On the other hand, unchecked activity of this complex causes branched-chain keto acid dehydrogenase kinase deficiency.
The BCKDH complex converts branched-chain amino acids into acyl-CoA derivatives, which after subsequent reactions are converted either into acetyl-CoA or succinyl-CoA that enter the citric acid cycle.[7]
Enzymes involved are
Maple syrup urine disease
In a rat model of maple syrup urine disease, acute administration of BCAAs increases DNA damage in the hippocampus region of the brain.[8] The nearby Figure shows the degradation pathway of BCAAs and specifically the key role of inadequate BCKDH in maple syrup urine disease. Chronic administration of BCAAs, compared to acute administration, increased DNA damage not only in the hippocampus but also in the striatum region of the brain.[8] Antioxidant treatment was able the prevent the DNA damage in these brain regions, suggesting that the BCAAs cause DNA damage through the production of oxidative stress.
Cell signaling
While most amino acids are oxidized in the liver, BCAAs are primarily oxidized in the
Role in diabetes mellitus type 2
In addition to cell signaling, the mTOR pathway also plays a role in beta cell growth leading to
Metformin is able to activate AMP kinase which phosphorylates proteins involved in the mTOR pathway, as well as leads to the progression of mTOR complex from its inactive state to its active state.[12] It is suggested that metformin acts as a competitive inhibitor to the amino acid leucine in the mTOR pathway.
Effects of BCAA supplementation on exercise
BCAAs have an insulin-like effect on
BCAAs reduce the levels of circulating
BCAA also inhibits tyrosine uptake in the brain (tyrosine being another aromatic amino acid, like tryptophan); the reduced uptake depresses catecholamine synthesis and release in the brain. Catecholamines are associated with enhanced physical performance. The simultaneous reductions in both catecholamine and serotonin synthesis may account for the relatively neutral effect of BCAA on physical performance.[17]
BCAAs are also found to reduce the increase in serum levels of
In addition, BCAA supplementation has been shown to decrease levels of creatine kinase in muscle cells post exercise. Creatine kinase is an indicator of muscle damage, and is responsible for transferring a phosphate group from ATP to create a phosphocreatine molecule.[19] BCAA supplementation has been shown to decrease levels of creatine kinase, leading to higher levels of intracellular ATP and a lessened sense of fatigue.[20] See also DOMS.
Research
Dietary BCAAs have been used in an attempt to treat some cases of hepatic encephalopathy.[21] They can have the effect of alleviating symptoms of hepatic encephalopathy, but there is no evidence they benefit mortality rates, nutrition, or overall quality of life as further research is necessary.[22]
Certain studies suggested a possible link between a high incidence of
Blood levels of the BCAAs are elevated in obese, insulin resistant humans and in mouse and rat models of diet-induced diabetes, suggesting the possibility that BCAAs contribute to the pathogenesis of obesity and diabetes.[24][25] BCAA-restricted diets improve glucose tolerance and promote leanness in normal weight mice,[26] restores insulin sensitivity and normal body weight to obese mice[27] and promotes insulin sensitivity in obese rats.[28] In lean and obese mice, these benefits of BCAA-restriction are mediated by isoleucine and valine, and not by restriction of leucine.[29]
Restriction of dietary BCAAs extends lifespan in flies,[30] while restriction of BCAAs in mice extends male lifespan and decreased frailty, but does not extend female lifespan.[31] In mice, dietary supplementation with BCAAs alone decreases lifespan and promotes obesity.[32] However, consumption of a BCAA-enriched essential amino acid supplement extends the lifespan of mice.[33]
See also
- Branched-chain alpha-keto acid dehydrogenase complex
- Maple syrup urine disease—excessive accumulation of BCAA in the organism
- BCKDK deficiency—insufficient levels of BCAA in the organism
References
- ^ Sowers S. "A Primer on Branched Chain Amino Acids" (PDF). Huntington College of Health Sciences. Archived from the original (PDF) on 28 August 2017. Retrieved 22 March 2011.
- PMID 15173434.
- ^ PMID 12242394.
- ^ hdl:1993/30476.
- PMID 19661225.
- ISBN 978-0-309-08525-0.
- PMID 19841271.
- ^ PMID 22560665.
- ^ PMID 16365087.
- ^ S2CID 16284975.
- ^ PMID 16365096.
- ^ PMID 22442749.
- ^ PMID 24893199.
- PMID 3471061.
- ^ S2CID 78093727.
- S2CID 20597074.
- ^ S2CID 1957988.
- PMID 6341752.
- ISBN 978-0-19-920828-9. Retrieved 6 December 2019.
- PMID 28870476.
- PMID 20581319.
- PMID 28518283.
- ^ PMID 21167830.
- PMID 25287287.
- PMID 19356713.
- PMID 27346343.
- PMID 29266268.
- PMID 27408778.
- PMID 33887198.
- PMID 30891588.
- PMID 33796866.
- PMID 31656947.
- PMID 20889128.
External links
- Branched-chain+amino+acids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Branched-chain amino acid degradation pathway
- Synthesic pathway in yeast (WikiPathways)