Calcium carbonate

| |
 | |

| |
| Names | |
|---|---|
| IUPAC name
Calcium carbonate
| |
| Other names | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
ECHA InfoCard
|
100.006.765 |
| EC Number |
|
| E number | E170 (colours) |
| KEGG | |
PubChem CID
|
|
RTECS number
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CaCO3 | |
| Molar mass | 100.0869 g/mol |
| Appearance | Fine white powder or colorless crystals; chalky taste |
| Odor | odorless |
| Density | 2.711 g/cm3 (calcite) 2.83 g/cm3 (aragonite) |
| Melting point | 1,339 °C (2,442 °F; 1,612 K) (calcite) 825 °C (1,517 °F; 1,098 K) (aragonite)[4][5] |
| Boiling point | decomposes |
| 0.013 g/L (25 °C)[1][2] | |
Solubility product (Ksp)
|
3.3×10−9[3] |
| Solubility in dilute acids | soluble |
| Acidity (pKa) | 9.0 |
| −3.82×10−5 cm3/mol | |
Refractive index (nD)
|
1.59 |
| Structure | |
| Trigonal | |
| 32/m | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
93 J/(mol·K)[6] |
Std enthalpy of (ΔfH⦵298)formation |
−1207 kJ/mol[6] |
| Pharmacology | |
| A02AC01 (WHO) A12AA04 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
6450 mg/kg (oral, rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[7] |
| Safety data sheet (SDS) | ICSC 1193 |
| Related compounds | |
Other anions
|
Calcium bicarbonate |
Other cations
|
|
Related compounds
|
Calcium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Calcium carbonate is a
Chemistry
Calcium carbonate shares the typical properties of other carbonates. Notably it
- reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water:
- CaCO3(s) + 2 H+(aq) → Ca2+(aq) + CO2(g) + H2O(l)
- releases carbon dioxide upon heating, called a quicklime, with reaction enthalpy178 kJ/mol:
- CaCO3(s) → CaO(s) + CO2(g)
Calcium carbonate reacts with water that is saturated with carbon dioxide to form the soluble calcium bicarbonate.
- CaCO3(s) + CO2(g) + H2O(l) → Ca(HCO3)2(aq)
This reaction is important in the
An unusual form of calcium carbonate is the hexahydrate ikaite, CaCO3·6H2O. Ikaite is stable only below 8 °C.
Preparation
The vast majority of calcium carbonate used in industry is extracted by mining or quarrying. Pure calcium carbonate (such as for food or pharmaceutical use), can be produced from a pure quarried source (usually marble).
Alternatively, calcium carbonate is prepared from
- CaO + H2O → Ca(OH)2
- Ca(OH)2 + CO2 → CaCO3 + H2O
In a laboratory, calcium carbonate can easily be crystallized from calcium chloride (CaCl2), by placing an aqueous solution of CaCl2 in a desiccator alongside ammonium carbonate [NH4]2CO3.[10] In the desiccator, ammonium carbonate is exposed to air and decomposes into ammonia, carbon dioxide, and water. The carbon dioxide then diffuses into the aqueous solution of calcium chloride, reacts with the calcium ions and the water, and forms calcium carbonate.
Structure
The thermodynamically stable form of CaCO3 under normal conditions is
Polymorphs
Calcium carbonate crystallizes in three
Crystal structure
The
Crystallization

All three polymorphs crystallize simultaneously from aqueous solutions under ambient conditions.[14] In additive-free aqueous solutions, calcite forms easily as the major product, while aragonite appears only as a minor product.
At high saturation, vaterite is typically the first phase precipitated, which is followed by a transformation of the vaterite to calcite.[19] This behavior seems to follow Ostwald's rule, in which the least stable polymorph crystallizes first, followed by the crystallization of different polymorphs via a sequence of increasingly stable phases.[20] However, aragonite, whose stability lies between those of vaterite and calcite, seems to be the exception to this rule, as aragonite does not form as a precursor to calcite under ambient conditions.[14]

Aragonite occurs in majority when the reaction conditions inhibit the formation of calcite and/or promote the nucleation of aragonite. For example, the formation of aragonite is promoted by the presence of magnesium ions,[21] or by using proteins and peptides derived from biological calcium carbonate.[22] Some polyamines such as cadaverine and Poly(ethylene imine) have been shown to facilitate the formation of aragonite over calcite.[14]
Selection by organisms
Organisms, such as molluscs and arthropods, have shown the ability to grow all three crystal polymorphs of calcium carbonate, mainly as protection (shells) and muscle attachments.[23] Moreover, they exhibit a remarkable capability of phase selection over calcite and aragonite, and some organisms can switch between the two polymorphs. The ability of phase selection is usually attributed to the use of specific macromolecules or combinations of macromolecules by such organisms.[24][25][26]
Occurrence
Geological sources
Calcite, aragonite and vaterite are pure calcium carbonate minerals. Industrially important source rocks which are predominantly calcium carbonate include limestone, chalk, marble and travertine.
Biological sources

Annelids in the family Lumbricidae, earthworms, possess a regionalization of the digestive track called calciferous glands, Kalkdrüsen, or glandes de Morren, that processes calcium and CO2 into calcium carbonate, which is later excreted into the dirt.[32] The function of these glands is unknown but is believed to serve as a CO2 regulation mechanism within the animals' tissues.[33] This process is ecologically significant, stabilizing the pH of acid soils.[34]
Extraterrestrial
Beyond Earth, strong evidence suggests the presence of calcium carbonate on
Geology
Carbonate is found frequently in geologic settings and constitutes an enormous carbon reservoir. Calcium carbonate occurs as aragonite, calcite and dolomite as significant constituents of the calcium cycle. The carbonate minerals form the rock types: limestone, chalk, marble, travertine, tufa, and others.

In warm, clear tropical waters
Where the
Carbonate compensation depth
The carbonate compensation depth (CCD) is the point in the ocean where the rate of precipitation of calcium carbonate is balanced by the rate of dissolution due to the conditions present. Deep in the ocean, the temperature drops and pressure increases. Increasing pressure also increases the solubility of calcium carbonate. Calcium carbonate is unusual in that its solubility increases with decreasing temperature.[37] The carbonate compensation depth ranges from 4,000 to 6,000 meters below sea level in modern oceans, and the various polymorphs (calcite, aragonite) have different compensation depths based on their stability.[38]
Role in taphonomy
Calcium carbonate can
Trilobite populations were once thought to have composed the majority of aquatic life during the Cambrian, due to the fact that their calcium carbonate-rich shells were more easily preserved than those of other species,[40] which had purely chitinous shells.
Uses
Construction
The main use of calcium carbonate is in the construction industry, either as a building material, or limestone
calcium carbonate (in limestone form) is no longer used for building purposes on its own, but only as a raw primary substance for building materials.Calcium carbonate is also used in the purification of iron from iron ore in a blast furnace. The carbonate is calcined in situ to give calcium oxide, which forms a slag with various impurities present, and separates from the purified iron.[42]
In the
It is also used as a raw material in the
Calcium carbonate in the form of
Fine ground calcium carbonate (GCC) is an essential ingredient in the microporous film used in
Calcium carbonate is widely used as an
Calcium carbonate is added to a wide range of trade and do it yourself adhesives, sealants, and decorating fillers.[46] Ceramic tile adhesives typically contain 70% to 80% limestone. Decorating crack fillers contain similar levels of marble or dolomite. It is also mixed with putty in setting stained glass windows, and as a resist to prevent glass from sticking to kiln shelves when firing glazes and paints at high temperature.[51][52][53][54]
In
Health and diet

Calcium carbonate is widely used medicinally as an inexpensive dietary calcium supplement for
Calcium carbonate is used in the production of calcium oxide as well as toothpaste and has seen a resurgence as a food preservative and color retainer, when used in or with products such as organic apples.[58]
Calcium carbonate is used therapeutically as phosphate binder in patients on maintenance
Excess calcium from supplements, fortified food, and high-calcium diets can cause
As a
Several calcium supplement formulations have been documented to contain the chemical element lead,[69] posing a public health concern.[70] Lead is commonly found in natural sources of calcium.[69]
Agriculture and aquaculture
Agricultural lime, powdered chalk or limestone, is used as a cheap method of neutralising acidic soil, making it suitable for planting, also used in aquaculture industry for pH regulation of pond soil before initiating culture.[71] There is interest in understanding whether or not it can affect pesticide adsorption and desorption in calcareous soil.[72]
Household cleaning
Calcium carbonate is a key ingredient in many household cleaning powders like Comet and is used as a scrubbing agent.
Pollution mitigation
In 1989, a researcher, Ken Simmons, introduced CaCO3 into the Whetstone Brook in Massachusetts.[73] His hope was that the calcium carbonate would counter the acid in the stream from acid rain and save the trout that had ceased to spawn. Although his experiment was a success, it did increase the amount of aluminium ions in the area of the brook that was not treated with the limestone. This shows that CaCO3 can be added to neutralize the effects of acid rain in river ecosystems. Currently calcium carbonate is used to neutralize acidic conditions in both soil and water.[74][75][76] Since the 1970s, such liming has been practiced on a large scale in Sweden to mitigate acidification and several thousand lakes and streams are limed repeatedly.[77]
Calcium carbonate is also used in flue-gas desulfurization applications eliminating harmful SO2 and NO2 emissions from coal and other fossil fuels burnt in large fossil fuel power stations.[74]
Calcination equilibrium
Calcination of limestone using charcoal fires to produce quicklime has been practiced since antiquity by cultures all over the world. The temperature at which limestone yields calcium oxide is usually given as 825 °C, but stating an absolute threshold is misleading. Calcium carbonate exists in equilibrium with calcium oxide and carbon dioxide at any temperature. At each temperature there is a partial pressure of carbon dioxide that is in equilibrium with calcium carbonate. At room temperature the equilibrium overwhelmingly favors calcium carbonate, because the equilibrium CO2 pressure is only a tiny fraction of the partial CO2 pressure in air, which is about 0.035 kPa.
At temperatures above 550 °C the equilibrium CO2 pressure begins to exceed the CO2 pressure in air. So above 550 °C, calcium carbonate begins to outgas CO2 into air. However, in a charcoal fired kiln, the concentration of CO2 will be much higher than it is in air. Indeed, if all the oxygen in the kiln is consumed in the fire, then the partial pressure of CO2 in the kiln can be as high as 20 kPa.[78]
The table shows that this partial pressure is not achieved until the temperature is nearly 800 °C. For the outgassing of CO2 from calcium carbonate to happen at an economically useful rate, the equilibrium pressure must significantly exceed the ambient pressure of CO2. And for it to happen rapidly, the equilibrium pressure must exceed total atmospheric pressure of 101 kPa, which happens at 898 °C.
Equilibrium pressure of CO2 over CaCO3 (P) versus temperature (T).[79] P (kPa) 0.055 0.13 0.31 1.80 5.9 9.3 14 24 34 51 72 80 91 101 179 901 3961 T (°C) 550 587 605 680 727 748 777 800 830 852 871 881 891 898 937 1082 1241
Solubility
With varying CO2 pressure

Calcium carbonate is poorly soluble in pure water (47 mg/L at normal atmospheric CO2 partial pressure as shown below).
The equilibrium of its solution is given by the equation (with dissolved calcium carbonate on the right):
CaCO3 ⇌ Ca2+ + CO2−3 Ksp = 3.7×10−9 to 8.7×10−9 at 25 °C
where the
HCO−3 ⇌ H+ + CO2−3 Ka2 = 5.61×10−11 at 25 °C
HCO−3 is known as the bicarbonate ion. Calcium bicarbonate is many times more soluble in water than calcium carbonate—indeed it exists only in solution.
Some of the HCO−3 combines with H+ in solution according to
H2CO3 ⇌ H+ + HCO−3 Ka1 = 2.5×10−4 at 25 °C
Some of the H2CO3 breaks up into water and dissolved carbon dioxide according to
H2O + CO2( aq) ⇌ H2CO3Kh = 1.70×10−3 at 25 °C
And dissolved carbon dioxide is in equilibrium with atmospheric carbon dioxide according to
PCO2/[CO2] = kH where kH = 29.76 atm/(mol/L) at 25 °C (Henry constant), PCO2 being the CO2 partial pressure.
For ambient air, PCO2 is around 3.5×10−4 atm (or equivalently 35 Pa). The last equation above fixes the concentration of dissolved CO2 as a function of PCO2, independent of the concentration of dissolved CaCO3. At atmospheric partial pressure of CO2, dissolved CO2 concentration is 1.2×10−5 moles per liter. The equation before that fixes the concentration of H2CO3 as a function of CO2 concentration. For [CO2] = 1.2×10−5, it results in [H2CO3] = 2.0×10−8 moles per liter. When [H2CO3] is known, the remaining three equations together with
| PCO2 (atm) | pH | [Ca2+] (mol/L) |
|---|---|---|
| 10−12 | 12.0 | 5.19×10−3 |
| 10−10 | 11.3 | 1.12×10−3 |
| 10−8 | 10.7 | 2.55×10−4 |
| 10−6 | 9.83 | 1.20×10−4 |
| 10−4 | 8.62 | 3.16×10−4 |
| 3.5×10−4 | 8.27 | 4.70×10−4 |
| 10−3 | 7.96 | 6.62×10−4 |
| 10−2 | 7.30 | 1.42×10−3 |
| 10−1 | 6.63 | 3.05×10−3 |
| 1 | 5.96 | 6.58×10−3 |
| 10 | 5.30 | 1.42×10−2 |
H2O ⇌ H+ + OH− K = 10−14 at 25 °C
(which is true for all aqueous solutions), and the fact that the solution must be electrically neutral, i.e., the overall charge of dissolved positive ions [Ca2+] + 2 [H+] must be cancelled out by the overall charge of dissolved negative ions [HCO−3] + [CO2−3] + [OH−], make it possible to solve simultaneously for the remaining five unknown concentrations (the previously mentioned form of the neutrality is valid only if calcium carbonate has been put in contact with pure water or with a neutral pH solution; in the case where the initial water solvent pH is not neutral, the balance is not neutral).
The adjacent table shows the result for [Ca2+] and [H+] (in the form of pH) as a function of ambient partial pressure of CO2 (Ksp = 4.47×10−9 has been taken for the calculation).
- At atmospheric levels of ambient CO2 the table indicates that the solution will be slightly alkaline with a maximum CaCO3 solubility of 47 mg/L.
- As ambient CO2 partial pressure is reduced below atmospheric levels, the solution becomes more and more alkaline. At extremely low PCO2, dissolved CO2, bicarbonate ion, and carbonate ion largely evaporate from the solution, leaving a highly alkaline solution of calcium hydroxide, which is more soluble than CaCO3. For PCO2 = 10−12 atm, the [Ca2+][OH−]2 product is still below the solubility product of Ca(OH)2 (8×10−6). For still lower CO2 pressure, Ca(OH)2 precipitation will occur before CaCO3 precipitation.
- As ambient CO2 partial pressure increases to levels above atmospheric, pH drops, and much of the carbonate ion is converted to bicarbonate ion, which results in higher solubility of Ca2+.
The effect of the latter is especially evident in day-to-day life of people who have hard water. Water in aquifers underground can be exposed to levels of CO2 much higher than atmospheric. As such water percolates through calcium carbonate rock, the CaCO3 dissolves according to one of the trends above. When that same water then emerges from the tap, in time it comes into equilibrium with CO2 levels in the air by outgassing its excess CO2. The calcium carbonate becomes less soluble as a result, and the excess precipitates as lime scale. This same process is responsible for the formation of
Two hydrated phases of calcium carbonate,
With varying pH, temperature and salinity: CaCO3 scaling in swimming pools
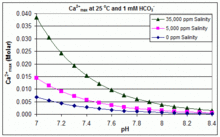
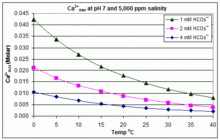
In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of sodium bicarbonate (NaHCO3) to the concentration of about 2 mmol/L as a buffer, then control of pH through use of HCl, NaHSO4, Na2CO3, NaOH or chlorine formulations that are acidic or basic. In this situation, dissolved inorganic carbon (total inorganic carbon) is far from equilibrium with atmospheric CO2. Progress towards equilibrium through outgassing of CO2 is slowed by
- the slow reaction
- limited aeration in a deep water column; and
- periodic replenishment of bicarbonate to maintain buffer capacity (often estimated through measurement of total alkalinity).
In this situation, the dissociation constants for the much faster reactions
- H2CO3 ⇌ H+ + HCO−3 ⇌ 2 H+ + CO2−3
allow the prediction of concentrations of each dissolved inorganic carbon species in solution, from the added concentration of HCO−3 (which constitutes more than 90% of Bjerrum plot species from pH 7 to pH 8 at 25 °C in fresh water).[82] Addition of HCO−3 will increase CO2−3 concentration at any pH. Rearranging the equations given above, we can see that [Ca2+] = Ksp/[CO2−3], and [CO2−3] = Ka2 [HCO−3]/[H+]. Therefore, when HCO−3 concentration is known, the maximum concentration of Ca2+ ions before scaling through CaCO3 precipitation can be predicted from the formula:
- [Ca2+]max = Ksp/Ka2 × [H+]/[HCO−3]
The solubility product for CaCO3 (Ksp) and the dissociation constants for the dissolved inorganic carbon species (including Ka2) are all substantially affected by temperature and salinity,[82] with the overall effect that [Ca2+]max increases from freshwater to saltwater, and decreases with rising temperature, pH, or added bicarbonate level, as illustrated in the accompanying graphs.
The trends are illustrative for pool management, but whether scaling occurs also depends on other factors including interactions with Mg2+, [B(OH)4]− and other ions in the pool, as well as supersaturation effects.[83][84] Scaling is commonly observed in electrolytic chlorine generators, where there is a high pH near the cathode surface and scale deposition further increases temperature. This is one reason that some pool operators prefer borate over bicarbonate as the primary pH buffer, and avoid the use of pool chemicals containing calcium.[85]
Solubility in a strong or weak acid solution
Solutions of
- In the case of a strong monoacid with decreasing acid concentration [A] = [A−], we obtain (with CaCO3 molar mass = 100 g/mol):
[A] (mol/L) 1 10−1 10−2 10−3 10−4 10−5 10−6 10−7 10−10 Initial pH 0.00 1.00 2.00 3.00 4.00 5.00 6.00 6.79 7.00 Final pH 6.75 7.25 7.75 8.14 8.25 8.26 8.26 8.26 8.27 Dissolved CaCO3
(g/L of acid)50.0 5.00 0.514 0.0849 0.0504 0.0474 0.0471 0.0470 0.0470
- where the initial state is the acid solution with no Ca2+ (not taking into account possible CO2 dissolution) and the final state is the solution with saturated Ca2+. For strong acid concentrations, all species have a negligible concentration in the final state with respect to Ca2+ and A− so that the neutrality equation reduces approximately to 2[Ca2+] = [A−] yielding [Ca2+] ≈ 0.5 [A−]. When the concentration decreases, [HCO−3] becomes non-negligible so that the preceding expression is no longer valid. For vanishing acid concentrations, one can recover the final pH and the solubility of CaCO3 in pure water.
- In the case of a weak monoacid (here we take acetic acid with pKa = 4.76) with decreasing total acid concentration [A] = [A−] + [AH], we obtain:
[A] (mol/L) [Ca2+] ≈ 0.5 [A−] 10−1 10−2 10−3 10−4 10−5 10−6 10−7 10−10 Initial pH 2.38 2.88 3.39 3.91 4.47 5.15 6.02 6.79 7.00 Final pH 6.75 7.25 7.75 8.14 8.25 8.26 8.26 8.26 8.27 Dissolved CaCO3
(g/L of acid)49.5 4.99 0.513 0.0848 0.0504 0.0474 0.0471 0.0470 0.0470
- For the same total acid concentration, the initial pH of the weak acid is less acid than the one of the strong acid; however, the maximum amount of CaCO3 which can be dissolved is approximately the same. This is because in the final state, the pH is larger than the pKa, so that the weak acid is almost completely dissociated, yielding in the end as many H+ ions as the strong acid to "dissolve" the calcium carbonate.
- The calculation in the case of phosphoric acid (which is the most widely used for domestic applications) is more complicated since the concentrations of the four dissociation states corresponding to this acid must be calculated together with [HCO−3], [CO2−3], [Ca2+], [H+] and [OH−]. The system may be reduced to a seventh degree equation for [H+] the numerical solution of which gives
[A] (mol/L) 1 10−1 10−2 10−3 10−4 10−5 10−6 10−7 10−10 Initial pH 1.08 1.62 2.25 3.05 4.01 5.00 5.97 6.74 7.00 Final pH 6.71 7.17 7.63 8.06 8.24 8.26 8.26 8.26 8.27 Dissolved CaCO3
(g/L of acid)62.0 7.39 0.874 0.123 0.0536 0.0477 0.0471 0.0471 0.0470
- where [A] = [H3PO4] + [H2PO−4] + [HPO2−4] + [PO3−4] is the total acid concentration. Thus phosphoric acid is more efficient than a monoacid since at the final almost neutral pH, the second dissociated state concentration [HPO2−4] is not negligible (see phosphoric acid).
See also


References
- ISBN 978-0-470-81638-7.
- ISBN 978-3-7643-6425-0.
- ISBN 978-0-07-238390-4.
- ^ "Occupational safety and health guideline for calcium carbonate" (PDF). US Dept. of Health and Human Services. Archived (PDF) from the original on 30 April 2011. Retrieved 31 March 2011.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 29 October 2018. Retrieved 29 October 2018.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ ISBN 978-0-618-94690-7.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0090". National Institute for Occupational Safety and Health (NIOSH).
- PMID 26318774.
- ^ "Precipitated Calcium Carbonate". Archived from the original on 11 January 2014. Retrieved 11 January 2014.
- PMID 25014563.
- ISSN 1528-7483.
- from the original on 1 December 2022. Retrieved 15 December 2022.
- )
- ^ S2CID 253707446.
- S2CID 146060934.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- ^ Negro, AD (1971). "Refinement of the crystal structure of aragonite" (PDF). American Mineralogist: Journal of Earth and Planetary Materials. 56: 768–772. Archived (PDF) from the original on 15 December 2022. Retrieved 15 December 2022 – via GeoScienceWorld.
- S2CID 206546317.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- ISBN 978-0-19-504977-0.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- from the original on 15 December 2022. Retrieved 15 December 2022.
- S2CID 220850117.
- ^ Russell, Daniel E . 17 February 2008. Retrieved December 31, 2010. "Helgustadir Iceland Spar Mine Archived 8 May 2019 at the Wayback Machine" mindat.org
- ^ Horne, Francis (23 October 2006). "How are seashells created?". Scientific American. Archived from the original on 19 March 2011. Retrieved 25 April 2012.
- ^ "Oyster shell calcium". WebMD. Retrieved 25 April 2012.
- ^ "Oyster Shell Calcium Carbonate". Caltron Clays & Chemicals. Archived from the original on 10 September 2013. Retrieved 25 April 2012.
- PMID 24898231.
- ^ "The Function of the Calciferous Glands of Earthworms". The company of biologists. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- ^ "Stable isotopes reveal that the calciferous gland of earthworms is a CO2-fixing organ". Science Direct. Archived from the original on 29 January 2012. Retrieved 5 February 2024.
- ^ "Ecological functions of earthworms in soil". eDepot. Archived from the original on 5 February 2024. Retrieved 5 February 2024.
- (PDF) from the original on 5 March 2016. Retrieved 7 January 2015.
- hdl:1893/17119. Archived(PDF) from the original on 29 July 2018. Retrieved 20 April 2018.
- .
- ^ Burton, Elizabeth (1990). "Carbonate compensation depth". Geochemistry: 73. Archived from the original on 22 December 2023. Retrieved 22 December 2023 – via Elsevier.
- ^ ISBN 978-0-253-33907-2.
- ISBN 978-0-309-66612-1. Archivedfrom the original on 1 January 2018. Retrieved 31 December 2017.
- ^ "Effects of Acid Rain". US Environmental Protection Agency. Archived from the original on 2 March 2015. Retrieved 14 March 2015.
- ^ "Blast Furnace". Science Aid. Archived from the original on 17 December 2007. Retrieved 30 December 2007.
- ^ Sfetcu, Nicolae (2 May 2014). Health & Drugs: Disease, Prescription & Medication. Nicolae Sfetcu.
- ^ McGinnis, R. A. Beet-Sugar Technology (2nd ed.). Beet Sugar Development Foundation. p. 178.
- ^ "Precipitated Calcium Carbonate uses". Archived from the original on 25 July 2014.
- ^ a b c d "Calcium Carbonate Powder". Reade Advanced Materials. 4 February 2006. Archived from the original on 22 February 2008. Retrieved 30 December 2007.
- ^ a b "Calcium carbonate in plastic applications". Imerys Performance Minerals. Archived from the original on 4 August 2008. Retrieved 1 August 2008.
- ^ "Why do calcium carbonate play an important part in Industrial". www.xintuchemical.com. Archived from the original on 7 October 2018. Retrieved 7 October 2018.
- ^ "precipitated calcium carbonate commodity price". www.dgci.be. Archived from the original on 7 October 2018. Retrieved 7 October 2018.
- (PDF) from the original on 21 September 2018. Retrieved 7 October 2018.
- ^ "Topic: Re: Can our calcium carbonate "waste" be utilized in other industries so we can divert it from landfills?". www.chemicalprocessing.com. 4 March 2010. Archived from the original on 23 March 2017. Retrieved 3 February 2021.
- ^ "Why do calcium carbonate play an important part in Industry?". www.xintuchemical.com. Archived from the original on 7 October 2018. Retrieved 3 February 2021.
- ^ "Calcium Carbonates / Calcite/ Limestone. CaCO3 | Rajasthan Minerals & Chemicals". www.rmcl.co.in. Archived from the original on 15 April 2021. Retrieved 3 February 2021.
- ^ "Calcium Carbonate". kamceramics.com. Archived from the original on 15 April 2021. Retrieved 3 February 2021.
- ^ "Ohio Historical Society Blog: Make It Shine". Ohio Historical Society. Archived from the original on 23 March 2012. Retrieved 2 June 2011.
- ^ "Calcium Carbonate". Medline Plus. National Institutes of Health. 1 October 2005. Archived from the original on 17 October 2007. Retrieved 30 December 2007.
- ISBN 978-0-8247-8044-9.
- ^ "Food Additives – Names Starting with C". Chemistry.about.com. 10 April 2012. Archived from the original on 16 October 2006. Retrieved 24 May 2012.
- PMID 17483976.
- S2CID 45802184.
- PMID 18450607.
- ^ "E-numbers: E170 Calcium carbonate". Food-Info.net. Archived from the original on 14 October 2022. Retrieved 19 April 2008. 080419 food-info.net
- ^ "Current EU approved additives and their E Numbers". UK Food Standards Agency. Archived from the original on 7 October 2010. Retrieved 27 October 2011.
- ^ "Listing of Food Additives Status Part I". US Food and Drug Administration. Archived from the original on 14 March 2013. Retrieved 27 October 2011.
- ^ "Standard 1.2.4 – Labelling of ingredients". Australia New Zealand Food Standards Code. Archived from the original on 2 September 2013. Retrieved 27 October 2011.
- ^ Holdstock, Lee. "Why go organic?". Real Bread Campaign. Soil Association Certification Limited. Archived from the original on 14 October 2022. Retrieved 3 April 2021.
- ^ "Bread and Flour Regulations 1998 A summary of responses to the consultation and Government Reply" (PDF). Department for Environment, Food and Rural Affairs. August 2013. Archived (PDF) from the original on 19 September 2021. Retrieved 9 April 2021.
- PMID 16177199.
- ^ from the original on 11 July 2021. Retrieved 11 July 2021.
- PMID 10989406.
- ISBN 978-3-527-61201-7.
- from the original on 18 August 2023. Retrieved 18 August 2023.
- ^ "Limestone Dispenser Fights Acid Rain in Stream". The New York Times. Associated Press. 13 June 1989. Archived from the original on 28 July 2018. Retrieved 27 July 2018.
- ^ a b "Environmental Uses for Calcium Carbonate". Congcal. 6 September 2012. Archived from the original on 4 January 2014. Retrieved 5 August 2013.
- from the original on 10 January 2018. Retrieved 28 August 2017.
- ^ Kircheis, Dan; Dill, Richard (2006). "Effects of low pH and high aluminum on Atlantic salmon smolts in Eastern Maine and liming project feasibility analysis" (reprinted at Downeast Salmon Federation). National Marine Fisheries Service and Maine Atlantic Salmon Commission.[permanent dead link]
- S2CID 129439066.
- ^ "Solvay Precipitated Calcium Carbonate: Production". Solvay. 9 March 2007. Archived from the original on 19 October 2007. Retrieved 30 December 2007.
- ^ ISBN 0-8493-0486-5.
- ^ "Selected Solubility Products and Formation Constants at 25 °C". California State University, Dominguez Hills. Archived from the original on 25 May 2006. Retrieved 7 June 2007.
- PMID 20039712.
- ^ a b Mook, W. (2000). "Chemistry of carbonic acid in water". Environmental Isotopes in the Hydrological Cycle: Principles and Applications (PDF). Paris: INEA/UNESCO. pp. 143–165. Archived from the original (PDF) on 18 March 2014. Retrieved 18 March 2014.
- ^ Wojtowicz, J. A. (1998). "Factors affecting precipitation of calcium carbonate" (PDF). Journal of the Swimming Pool and Spa Industry. 3 (1): 18–23. Archived from the original (PDF) on 18 March 2014. Retrieved 18 March 2014.
- ^ Wojtowicz, J. A. (1998). "Corrections, potential errors, and significance of the saturation index" (PDF). Journal of the Swimming Pool and Spa Industry. 3 (1): 37–40. Archived from the original (PDF) on 24 August 2012. Retrieved 18 March 2014.
- ^ Birch, R. G. (2013). "BABES: a better method than "BBB" for pools with a salt-water chlorine generator" (PDF). scithings.id.au. Archived (PDF) from the original on 15 April 2021. Retrieved 11 October 2020.

