Source: Wikipedia, the free encyclopedia.
Capsazepine
Names
Preferred IUPAC name
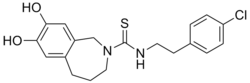
N -[2-(4-Chlorophenyl)ethyl]-7,8-dihydroxy-1,3,4,5-tetrahydro-2H -2-benzazepine-2-carbothioamide
Other names
N -(4-Chlorophenethyl)-7,8-dihydroxy-1,3,4,5-tetrahydro-2H -benzo[c ]azepine-2-carbothioamideN -(4-Chlorophenethyl)-7,8-dihydroxy-4,5-dihydro-1H -benzo[c ]azepine-2(3H )-carbothioamide
Identifiers
ChEMBL
ChemSpider
UNII
InChI=1S/C19H21ClN2O2S/c20-16-5-3-13(4-6-16)7-8-21-19(25)22-9-1-2-14-10-17(23)18(24)11-15(14)12-22/h3-6,10-11,23-24H,1-2,7-9,12H2,(H,21,25)
Y Key: DRCMAZOSEIMCHM-UHFFFAOYSA-N
Y InChI=1/C19H21ClN2O2S/c20-16-5-3-13(4-6-16)7-8-21-19(25)22-9-1-2-14-10-17(23)18(24)11-15(14)12-22/h3-6,10-11,23-24H,1-2,7-9,12H2,(H,21,25)
Key: DRCMAZOSEIMCHM-UHFFFAOYAH
Properties
C19 H21 ClN2 O2 S
Molar mass
376.9 g/mol
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Capsazepine is a synthetic antagonist of capsaicin .[1] TRPV ion channels .
Pharmacology Capsazepine blocks the painful sensation of heat caused by capsaicin (the active ingredient of
Development Capsazepine was discovered by a research group working for Novartis .[1] [7]
Use in biotechnology By incorporation of an azobenzene unit, a photoswitchable version of capsazepine (AC4) was developed in 2013 that allows for optical control of TRPV1 channels with light.[8] [9]
See also References
TRPA
TRPC
TRPM
TRPML
TRPP
TRPV
Activators
2-APB 5',6'-EET 9-HODE 9-oxoODE 12S-HETE 12S-HpETE 13-HODE 13-oxoODE 20-HETE )
Allicin (garlic )AM404 Anandamide Bisandrographolide (Andrographis paniculata )
Cannabidiol (cannabis )Cannabidivarin (cannabis )Capsaicin (chili pepper )Carvacrol (oregano , thyme , pepperwort , wild bergamot , others)DHEA Diacyl glycerol Dihydrocapsaicin (chili pepper )Estradiol Eugenol (basil , clove )Evodiamine (Euodia ruticarpa Gingerols (ginger )GSK1016790A Heat Hepoxilin A3
Hepoxilin B3
Homocapsaicin (chili pepper )Homodihydrocapsaicin (chili pepper )Incensole (incense )Lysophosphatidic acid Low pH (acidic conditions)
Menthol (mint )N-Arachidonoyl dopamine N-Oleoyldopamine N-Oleoylethanolamide Nonivamide (PAVA) (PAVA spray )Nordihydrocapsaicin (chili pepper )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) Phenylacetylrinvanil Phorbol esters (e.g., 4α-PDD )Piperine (black pepper , long pepper )Polygodial (Dorrigo pepper )Probenecid Protons RhTx Rutamarin (Ruta graveolens Resiniferatoxin (RTX) (Euphorbia resinifera /pooissonii Shogaols (ginger , Sichuan and melegueta peppers )Tetrahydrocannabivarin (cannabis )Thymol (thyme , oregano )Tinyatoxin (Euphorbia resinifera /pooissonii Tramadol Vanillin (vanilla )Zucapsaicin Blockers