Carbocation


A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication C
2H2+
4).[1]
Until the early 1970s, all carbocations were called carbonium ions.
Definitions
According to the
Since the late 1990s, most textbooks have stopped using the term carbonium ion for the classical three-coordinate carbocation. However, some university-level textbooks continue to use the term carbocation as if it were synonymous with carbenium ion,[6][7] or discuss carbocations with only a fleeting reference to the older terminology of carbonium ions[8] or carbenium and carbonium ions.[9] One textbook retains the older name of carbonium ion for carbenium ion to this day, and uses the phrase hypervalent carbonium ion for CH+
5.[10]
A carbocation with a two-coordinate positive carbon derived from formal removal of a hydride ion (H−) from an alkene is known as a vinyl cation. In the absence of geometric constraints, most substituted vinyl cations carry the formal positive charge on an sp-hydridized carbon atom of linear geometry. A two-coordinate approximately sp2-hybridized cation resulting from the formal removal of a hydride ion from an arene is termed an aryl cation. These carbocations are relatively unstable (aryl cations especially so) and are infrequently encountered. Hence, they are frequently omitted from introductory and intermediate level textbooks. The IUPAC definition stipulates that carbocations are even-electron species; hence, radical cations like CH•+
4 that are frequently encountered in mass spectrometry are not considered to be carbocations.
History
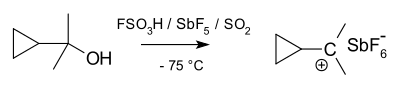
The history of carbocations dates back to 1891 when G. Merling
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol gives deep-yellow solutions in concentrated sulfuric acid. Triphenylmethyl chloride similarly formed orange complexes with aluminium and tin chlorides. In 1902, Adolf von Baeyer recognized the salt-like character of the compounds formed. The trityl carbocation (shown below) is a stable carbocationic system that has been used as homogeneous organocatalyst in organic synthesis,[13] for example in the form of trityl hexafluorophosphate.[14]
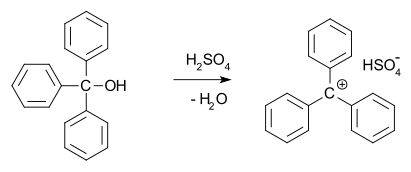
He dubbed the relationship between color and salt formation halochromy, of which malachite green is a prime example.
Carbocations are
The first
In 1962,
Structure and properties
Carbonium ions can be thought of as protonated alkanes. Although alkanes are usually considered inert, under superacid conditions (e.g., HF·SbF5), the C-H sigma bond can act as a donor to H+. This results in a species that contains a 3c-2e bond between a carbon and two hydrogen atoms, a type of bonding common in boron chemistry, though relatively uncommon for carbon. As an alternative view point, the 3c-2e bond of carbonium ions could be considered as a molecule of H2 coordinated to a carbenium ion (see below). Indeed, carbonium ions frequently decompose by loss of molecular hydrogen to form the corresponding carbenium ion. Structurally, the methanium ion CH+5 is computed to have a minimum energy structure of Cs symmetry. However, the various possible structures of the ion are close in energy and separated by shallow barriers. Hence, the structure of the ion is often described as fluxional. Although there appear to be five bonds to carbon in carbonium ions, they are not hypervalent, as the electron count around the central carbon is only eight, on account of the 3c-2e bond.
In contrast, at least in a formal sense, carbenium ions are derived from the protonation (addition of H+) or alkylation (addition of R+) of a carbene or alkene. Thus, in at least one of their resonance depictions, they possess a carbon atom bearing a formal positive charge that is surrounded by a sextet of electrons (six valence electrons) instead of the usual octet required to fill the valence shell of carbon (octet rule). Therefore, carbenium ions (and carbocations in general) are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge. In accord with VSEPR and Bent's rule, unless geometrically constrained to be pyramidal (e.g., 1-adamantyl cation), 3-coordinate carbenium ions are usually trigonal planar, with a pure p character empty orbital as its lowest unoccupied molecular orbital (LUMO) and CH/CC bonds formed from C(sp2) orbitals. A prototypical example is the methyl cation, CH+3. For the same reasons, carbocations that are 2-coordinate (vinyl cations) are generally linear in geometry, with CH/CC bonds formed from C(sp) orbitals.

Alkyl-substituted carbocations follow the order 3° > 2° > 1° > methyl in stability, as can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for (CH3)3C+, (CH3)2CH+, CH3CH+2, and CH+3).
The stabilization by alkyl groups is explained by hyperconjugation.[30] The donation of electron density from a β C-H or C-C bond into the unoccupied p orbital of the carbocation (a σCH/CC → p interaction) allows the positive charge to be delocalized.
Based on hydride ion affinity, the parent vinyl cation is less stable than even a primary sp2-hybridized carbocation, while an α alkyl-substituted vinyl cation has a stability that is comparable to the latter. Hence, vinyl cations are relatively uncommon intermediates. They can be generated by the ionization of a vinyl electrophile, provided the leaving group is sufficiently good (e.g., TfO−, IPh, or N2). They have been implicated as intermediates in some vinyl substitution reactions (designated as SN1(vinyl)) and as intermediates in the electrophilic addition reactions of arylalkynes. With the exception of the parent vinyl cation, which is believed to be a bridged species, and geometrically constrained cyclic vinyl cations, most vinyl cations take on sp hybridization and are linear.
Aryl cations are more unstable than vinyl cations, due to the ring-enforced distortion to a nonlinear geometry and approximately sp2-character of the unoccupied orbital. Only N2 in aryldiazonium salts is a good enough leaving group for the chemical generation of aryl cations.[31]
Alkynyl cations are extremely unstable, much less stable than even CH+
3 (hydride ion affinity 386 kcal/mol versus 312 kcal/mol for CH+
3) and cannot be generated by purely chemical means. They can, however, be generated radiochemically via the beta decay of tritium:[32]

In terms of reactivity, carbocations are susceptible to attack by nucleophiles, like water, alcohols, carboxylates, azide, and halide ions, to form the addition product. Strongly basic nucleophiles, especially hindered ones, favor elimination over addition. Because even weak nucleophiles will react with carbocations, most can only be directly observed or isolated in non-nucleophilic media like superacids.[33]

Carbocations typically undergo

A carbocation may be stabilized by
The stability order of carbocations, from most stable to least stable as reflected by hydride ion affinity (HIA) values, are as follows (HIA values in kcal/mol in parentheses):
| Carbocation | c-C7H+7 (most stable) | (C6H5)3C+ | c-C3H+3 | (C6H5)2CH+ | 2-norbornyl+ | t-C4H+9 | C6H5CH+2 | i-C3H+7 |
|---|---|---|---|---|---|---|---|---|
| HIA (kcal/mol) | 201 | 215 | 221 | 222 | 231 | 231 | 234 | 246 |
| Carbocation | c-C3H5CH+2 | CH2=CH−CH+2 | c-C5H+5 | CH≡C−CH+2 | C2H+5 | C2H+3 | C6H+5 | CH+3 (least stable) |
| HIA (kcal/mol) | 249 | 256 | 258 | 270 | 273 | 287 | 298 | 312 |
As noted in the history section, the
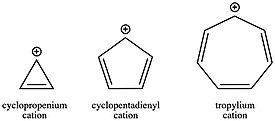
The effect of hyperconjugation is strongly stabilizing for carbocations: hyperconjugation with alkyl substituents is often as stabilizing or even more so than conjugation with a π system. Although conjugation to unsaturated groups results in significant stabilization by the mesomeric effect (resonance), the benefit is partially offset by the presence of a more electronegative sp2 or sp carbon next to the carbocationic center. Thus, as reflected by hydride ion affinities, a secondary carbocation is more stabilized than the allyl cation, while a tertiary carbocation is more stabilized than the benzyl cation — results that may seem counterintuitive on first glance.
Oxocarbenium and iminium ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the formal positive charge on an oxygen or nitrogen atom, respectively.
Non-classical ions
Some carbocations such as the 2-norbornyl cation exhibit more or less symmetrical three-center two-electron bonding. Such structures, referred to as non-classical carbocations, involve the delocalization of the bonds involved in the σ-framework of the molecule, resulting in C–C and C–H bonds of fractional bond order.[39][40] This delocalization results in additional stabilization of the cation. For instance, depicted as a classical carbenium ion, 2-norbornyl cation appears to be a secondary carbocation. However, it is more stable than a typical "secondary" carbocation, being roughly as stable as a tertiary carbocation like t-butyl cation, according to hydride ion affinity.
The existence of non-classical carbocations was once the subject of great controversy. On opposing sides were
Specific carbocations
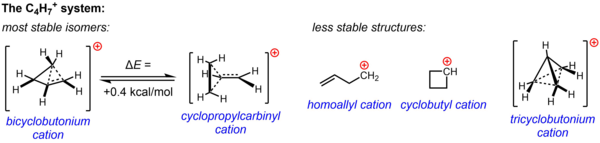
A non-classical structure for C
4H+
7 is supported by substantial experimental evidence from solvolysis experiments and NMR studies conducted in non-nucleophilic media. One or both of two structures, the cyclopropylcarbinyl cation and the bicyclobutonium cation, were invoked to account for the observed reactivity in various experiments, while the NMR data point to a highly fluxional system that undergoes rapid rearrangement to give an averaged spectrum consisting of only two 13C NMR signals, even at temperatures as low as −132 °C. Computationally, it was confirmed that the energetic landscape of the C
4H+
7 system is very flat, and that the two isomers postulated based on experimental data are very close in energy, the bicyclobutonium structure being computed to be just 0.4 kcal/mol more stable than the cyclopropylcarbinyl structure. In the solution phase (SbF5·SO2ClF·SO2F2, with SbF–
6 as the counterion), the bicyclobutonium structure predominates over the cyclopropylcarbinyl structure in a 84:16 ratio at −61 °C.
Three other possible structures, two classical structures (the homoallyl cation and cyclobutyl cation) and a more highly delocalized non-classical structure (the tricyclobutonium ion), are now known to be less stable isomers (or merely a transition state rather than an energy minimum in the case of the cyclobutyl cation).[44]
Substituted cyclopropylcarbinyl cations have also been studied by NMR:[45][46]
In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups, indicating that the
In terms of bent bond theory, this preference is explained by assuming favorable orbital overlap between the filled cyclopropane bent bonds and the empty p-orbital.[47]
Pyramidal carbocation
| Pyramidal Carbocations | ||
|---|---|---|
|
|
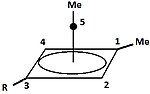
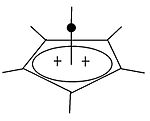
Besides the classical and non-classical carbocations, a third class can be distinguished: pyramidal carbocations. In these ions, a single carbon atom hovers over a four- or five-sided polygon, in effect forming a pyramid. The square pyramidal ion will carry a charge of +1, the Pentagonal pyramidal ion will carry +2.
A stable hexagonal-pyramidal configuration of tropylium trication, (C7H7)3+, has been also predicted.[48] In this case, the coordination number of carbon reaches seven. The crystal structure of [C6(CH3)6][SbF6]2·HSO3F confirms the pentagonal-pyramidal shape of the hexamethylbenzene dication.[49] |
| An example of the monovalent carbocation | An example of the divalent carbocation |
See also
References
- .
- ISBN 978-0-387-95468-4.
- .
- ISSN 1365-3075.
- ISBN 978-0967855097, retrieved 2018-11-03
- ISBN 978-0-534-37617-8.
- OCLC 1007924903.)
{{cite book}}: CS1 maint: location missing publisher (link - ISBN 978-0-13-140748-0.
- ISBN 978-0-19-850346-0.
- ISBN 978-0-7637-0413-1.
- ISSN 0365-9496.
- .
- .
- ISBN 0471936235.
- ISSN 0096-4085.
- ^ Meerwein, H.; Emster, K. van (1922). "About the equilibrium isomerism between bornyl chloride isobornyl chloride and camphene chlorohydrate". Berichte. 55: 2500.
- .
- .
- .
- .
- .
- ISBN 978-1891389313.
- OCLC 286483846.
- ISSN 0002-7863.
- OCLC 154040953.
- OCLC 14214254.
- OCLC 154040953.
- ISSN 0002-7863.
- ISSN 0022-3263.
- OCLC 154040953.
- OCLC 154040953.
- ISSN 0002-7863.
- OCLC 154040953.
- OCLC 154040953.
- ^ S2CID 206549219.
- OCLC 154040953.
- S2CID 222190636.
- OCLC 154040953.
- ^ Strictly speaking, the hyperconjugative stabilization of alkyl-substituted carbocations is a type of three-center bonding. Geometrically, the C–H bonds involved in hyperconjugation are observed (or computed) to "lean" slightly toward the carbocationic center as a result (that is, the +C–C–H bond angle decreases somewhat). Nevertheless, the hydrogen atom is still primarily bonded to the carbon α to the cationic carbon. To qualify as a non-classical carbocation, the two-electron three-center bond needs to feature a group equally (or nearly equally) bonded to two electron-deficient centers. In practice, there is a continuum of possible bonding schemes, ranging from slight involvement of a neighboring group (weak hyperconjugation) to equal sharing of a group between adjacent centers (fully non-classical bonding).
- OCLC 154040953.
- .
- ^ George A. Olah - Nobel Lecture
- ISSN 0002-7863.
- PMID 18570420.
- .
- .
- ^ Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A (2nd ed.).
- S2CID 13960338.
- PMID 27885766.
External links
 Media related to Carbocations at Wikimedia Commons
Media related to Carbocations at Wikimedia Commons- Press Release The 1994 Nobel Prize in Chemistry". Nobelprize.org. 9 Jun 2010

![{\displaystyle {\ce {RC#CT -> [RC#C^3 He]+ + e-}}+{\bar {\nu }}_{e}\longrightarrow {\ce {RC#C+ + ^{3}He + e-}}+{\bar {\nu }}_{e}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b407467d1926148009140dc78110535945708543)