Carbon monoxide
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Carbon monoxide
| |||
| Other names
Carbonic oxide gas
Carbon protoxide Oxide of carbon Protoxide of carbon Carbonous oxide Carbonous acid gas Carbon(II) oxide Breath of carbon Oxygenated carbon Carbate Carbonyl Water gas Hydrocarbon gas Fuel gas Rauchgas Carbonic inflammable air Heavy inflammable air White damp Fire Damp Powder Gas Illuminating gas Dowson gas Mond gas Power gas Producer gas Blast furnace gas Coal gas Phlogiston Car gas | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| 3587264 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
ECHA InfoCard
|
100.010.118 | ||
| EC Number |
| ||
| 421 | |||
| KEGG | |||
| MeSH | Carbon+monoxide | ||
PubChem CID
|
|||
RTECS number
|
| ||
| UNII | |||
| UN number | 1016 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CO | |||
| Molar mass | 28.010 g·mol−1 | ||
| Appearance | Colorless | ||
| Odor | Odorless | ||
| Density |
| ||
| Melting point | −205.02 °C (−337.04 °F; 68.13 K) | ||
| Boiling point | −191.5 °C (−312.7 °F; 81.6 K) | ||
| 27.6 mg/L (25 °C) | |||
| Solubility | soluble in ammonium hydroxide, benzene
| ||
Henry's law
constant (kH) |
1.04 atm·m3/mol | ||
| −9.8·10−6 cm3/mol | |||
Refractive index (nD)
|
1.0003364 | ||
| 0.122 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
29.1 J/(K·mol) | ||
Std molar
entropy (S⦵298) |
197.7 J/(K·mol) | ||
Std enthalpy of (ΔfH⦵298)formation |
−110.5 kJ/mol | ||
Std enthalpy of (ΔcH⦵298)combustion |
−283.0 kJ/mol | ||
| Pharmacology | |||
| V04CX08 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Poisonous by inhalation[1] | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H220, H331, H360, H372, H420 | |||
| P201, P202, P210, P251, P260, P261, P264, P270, P281, P304+P340, P308+P313, P311, P314, P321, P377, P381, P403, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −191 °C (−311.8 °F; 82.1 K) | ||
| 609 °C (1,128 °F; 882 K) | |||
Explosive limits
|
12.5–74.2% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
| ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits):[1] | |||
PEL (Permissible)
|
TWA 50 ppm (55 mg/m3) | ||
REL (Recommended)
|
| ||
IDLH (Immediate danger) |
1200 ppm | ||
| Safety data sheet (SDS) | ICSC 0023 | ||
| Related compounds | |||
Other anions
|
Carbon monosulfide | ||
Other cations
|
Silicon monoxide Germanium monoxide Tin(II) oxide Lead(II) oxide | ||
Related carbon oxides
|
Carbon dioxide Carbon suboxide Oxocarbons | ||
| Supplementary data page | |||
| Carbon monoxide (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.[5]
The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels.[6] Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change.[7]
Carbon monoxide has important biological roles across phylogenetic kingdoms. It is produced by many organisms, including humans. In mammalian physiology, carbon monoxide is a classical example of
N2.History
Prehistory
Humans have maintained a complex relationship with carbon monoxide since first learning to control fire circa 800,000 BC. Early humans probably discovered the toxicity of carbon monoxide poisoning upon introducing fire into their dwellings. The early development of
Ancient history
Early civilizations developed mythological tales to explain the origin of fire, such as Prometheus from Greek mythology who shared fire with humans. Aristotle (384–322 BC) first recorded that burning coals produced toxic fumes. Greek physician Galen (129–199 AD) speculated that there was a change in the composition of the air that caused harm when inhaled, and many others of the era developed a basis of knowledge about carbon monoxide in the context of coal fume toxicity. Cleopatra may have died from carbon monoxide poisoning.[8]
Pre-industrial revolution
Georg Ernst Stahl mentioned carbonarii halitus in 1697 in reference to toxic vapors thought to be carbon monoxide. Friedrich Hoffmann conducted the first modern scientific investigation into carbon monoxide poisoning from coal in 1716. Herman Boerhaave conducted the first scientific experiments on the effect of carbon monoxide (coal fumes) on animals in the 1730s.[8]
Joseph Priestley is considered to have first synthesized carbon monoxide in 1772. Carl Wilhelm Scheele similarly isolated carbon monoxide from charcoal in 1773 and thought it could be the carbonic entity making fumes toxic. Torbern Bergman isolated carbon monoxide from oxalic acid in 1775. Later in 1776, the French chemist de Lassone produced CO by heating zinc oxide with coke, but mistakenly concluded that the gaseous product was hydrogen, as it burned with a blue flame. In the presence of oxygen, including atmospheric concentrations, carbon monoxide burns with a blue flame, producing carbon dioxide. Antoine Lavoisier conducted similar inconclusive experiments to Lassone in 1777. The gas was identified as a compound containing carbon and oxygen by William Cruickshank in 1800.[8][9]
Advent of industrial chemistry
Carbon monoxide gained recognition as an essential reagent in the 1900s.
Physical and chemical properties
Carbon monoxide is the simplest
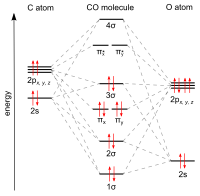
The carbon and oxygen are connected by a triple bond that consists of a net two pi bonds and one sigma bond. The bond length between the carbon atom and the oxygen atom is 112.8 pm.[11][12] This bond length is consistent with a triple bond, as in molecular nitrogen (N2), which has a similar bond length (109.76 pm) and nearly the same molecular mass. Carbon–oxygen double bonds are significantly longer, 120.8 pm in formaldehyde, for example.[13] The boiling point (82 K) and melting point (68 K) are very similar to those of N2 (77 K and 63 K, respectively). The bond-dissociation energy of 1072 kJ/mol is stronger than that of N2 (942 kJ/mol) and represents the strongest chemical bond known.[14]
The
| Temperature (°C) | Temperature (K) | Density (kg/m3) | Specific heat (J/g °C) | Dynamic viscosity (cg/m s) | Kinematic viscosity (cm2/s) | Thermal conductivity (cW/m °C) | Thermal diffusivity (cm2/s) | Prandtl number |
|---|---|---|---|---|---|---|---|---|
| -73.15 | 200 | 1.6888 | 1.045 | 1.27 | 0.0752 | 1.7 | 0.0963 | 0.781 |
| -53.15 | 220 | 1.5341 | 1.044 | 1.37 | 0.0893 | 1.9 | 0.119 | 0.753 |
| -33.15 | 240 | 1.4055 | 1.043 | 1.47 | 0.105 | 2.06 | 0.141 | 0.744 |
| -13.15 | 260 | 1.2967 | 1.043 | 1.57 | 0.121 | 2.21 | 0.163 | 0.741 |
| 6.85 | 280 | 1.2038 | 1.042 | 1.66 | 0.138 | 2.36 | 0.188 | 0.733 |
| 26.85 | 300 | 1.1233 | 1.043 | 1.75 | 0.156 | 2.5 | 0.213 | 0.73 |
| 46.85 | 320 | 1.0529 | 1.043 | 1.84 | 0.175 | 2.63 | 0.239 | 0.73 |
| 66.85 | 340 | 0.9909 | 1.044 | 1.93 | 0.195 | 2.78 | 0.269 | 0.725 |
| 86.85 | 360 | 0.9357 | 1.045 | 2.02 | 0.216 | 2.91 | 0.298 | 0.725 |
| 106.85 | 380 | 0.8864 | 1.047 | 2.1 | 0.237 | 3.05 | 0.329 | 0.729 |
| 126.85 | 400 | 0.8421 | 1.049 | 2.18 | 0.259 | 3.18 | 0.36 | 0.719 |
| 176.85 | 450 | 0.7483 | 1.055 | 2.37 | 0.317 | 3.5 | 0.443 | 0.714 |
| 226.85 | 500 | 0.67352 | 1.065 | 2.54 | 0.377 | 3.81 | 0.531 | 0.71 |
| 276.85 | 550 | 0.61226 | 1.076 | 2.71 | 0.443 | 4.11 | 0.624 | 0.71 |
| 326.85 | 600 | 0.56126 | 1.088 | 2.86 | 0.51 | 4.4 | 0.721 | 0.707 |
| 376.85 | 650 | 0.51806 | 1.101 | 3.01 | 0.581 | 4.7 | 0.824 | 0.705 |
| 426.85 | 700 | 0.48102 | 1.114 | 3.15 | 0.655 | 5 | 0.933 | 0.702 |
| 476.85 | 750 | 0.44899 | 1.127 | 3.29 | 0.733 | 5.28 | 1.04 | 0.702 |
| 526.85 | 800 | 0.42095 | 1.14 | 3.43 | 0.815 | 5.55 | 1.16 | 0.705 |
Bonding and dipole moment
Carbon and oxygen together have a total of 10
The molecule is therefore asymmetric: oxygen has more electron density than carbon and is also slightly positively charged compared to carbon being negative. By contrast, the

Carbon monoxide has a computed fractional bond order of 2.6, indicating that the "third" bond is important but constitutes somewhat less than a full bond.[19] Thus, in valence bond terms, –C≡O+ is the most important structure, while :C=O is non-octet, but has a neutral formal charge on each atom and represents the second most important resonance contributor. Because of the lone pair and divalence of carbon in this resonance structure, carbon monoxide is often considered to be an extraordinarily stabilized carbene.[20] Isocyanides are compounds in which the O is replaced by an NR (R = alkyl or aryl) group and have a similar bonding scheme.
If carbon monoxide acts as a ligand, the polarity of the dipole may reverse with a net negative charge on the oxygen end, depending on the structure of the coordination complex.[21] See also the section "Coordination chemistry" below.
Bond polarity and oxidation state
Theoretical and experimental studies show that, despite the greater electronegativity of oxygen, the dipole moment points from the more-negative carbon end to the more-positive oxygen end.
The oxidation state of carbon in carbon monoxide is +2 in each of these structures. It is calculated by counting all the bonding electrons as belonging to the more electronegative oxygen. Only the two non-bonding electrons on carbon are assigned to carbon. In this count, carbon then has only two valence electrons in the molecule compared to four in the free atom.
Occurrence
Carbon monoxide occurs in various natural and artificial environments. Photochemical degradation of plant matter for example generates an estimated 60 million tons/year.
| Concentration (ppmv[a]) | Source |
|---|---|
| 0.1 | Natural atmosphere level (MOPITT)[28] |
| 0.5–5 | Average level in homes[29] |
| 5–15 | Near properly-adjusted gas stoves in homes, modern vehicle exhaust emissions[30][citation needed] |
| 17 | Atmosphere of Venus |
| 100–200 | Exhaust from automobiles in the Mexico City central area in 1975[31] |
| 700 | Atmosphere of Mars |
| <1,000 | Car exhaust fumes after passing through catalytic converter[32] |
| 5,000 | Exhaust from a home wood fire[33] |
| 30,000–100,000 | Undiluted warm car exhaust without a catalytic converter[32] |
| |
Atmospheric presence

Carbon monoxide (CO) is present in small amounts (about 80 ppb) in the
Carbon monoxide has an indirect effect on
Due to its long lifetime in the mid-troposphere, carbon monoxide is also used as a tracer for pollutant plumes.[39]
Pollution
Urban pollution
Carbon monoxide is a temporary atmospheric pollutant in some urban areas, chiefly from the exhaust of internal combustion engines (including vehicles, portable and back-up generators, lawnmowers, power washers, etc.), but also from incomplete combustion of various other fuels (including wood, coal, charcoal, oil, paraffin, propane, natural gas, and trash).
Large CO pollution events can be observed from space over cities.[40]
Role in ground level ozone formation
Carbon monoxide is, along with
- CO + 2O2 + hν → CO2 + O3
(where hν refers to the photon of light absorbed by the NO2 molecule in the sequence)
Although the creation of NO2 is the critical step leading to low level ozone formation, it also increases this ozone in another, somewhat mutually exclusive way, by reducing the quantity of NO that is available to react with ozone.[42]
Indoor pollution
In closed environments, the concentration of carbon monoxide can rise to lethal levels. On average, 170 people in the United States die every year from carbon monoxide produced by non-automotive consumer products.[43] These products include malfunctioning fuel-burning appliances such as furnaces, ranges, water heaters, and gas and kerosene room heaters; engine-powered equipment such as portable generators (and cars left running in attached garages); fireplaces; and charcoal that is burned in homes and other enclosed areas. Many deaths have occurred during power outages due to severe weather such as Hurricane Katrina[43] and the 2021 Texas power crisis.[44]
Mining
Miners refer to carbon monoxide as "
Astronomy
Beyond Earth, carbon monoxide is the second-most common diatomic molecule in the
Beta Pictoris, the second brightest star in the constellation Pictor, shows an excess of infrared emission compared to normal stars of its type, which is caused by large quantities of dust and gas (including carbon monoxide)[48][49] near the star.
In the atmosphere of Venus carbon monoxide occurs as a result of the photodissociation of carbon dioxide by electromagnetic radiation of wavelengths shorter than 169 nm. It has also been identified spectroscopically on the surface of Neptune's moon Triton.[50]
Solid carbon monoxide is a component of comets.[51] The volatile or "ice" component of Halley's Comet is about 15% CO.[52] At room temperature and at atmospheric pressure, carbon monoxide is actually only metastable (see Boudouard reaction) and the same is true at low temperatures where CO and CO
2 are solid, but nevertheless it can exist for billions of years in comets. There is very little CO in the atmosphere of Pluto, which seems to have been formed from comets. This may be because there is (or was) liquid water inside Pluto.
Carbon monoxide can react with water to form carbon dioxide and hydrogen:
- CO + H2O → H
2 + CO
2
This is called the
- CO + H2O → HCOOH
These reactions can take place in a few million years even at temperatures such as found on Pluto.[53]
Chemistry
Carbon monoxide has a wide range of functions across all disciplines of chemistry. The four premier categories of reactivity involve
Coordination chemistry
Most metals form
- Ni + 4 CO → Ni(CO)4 (1 bar, 55 °C)
For this reason, nickel in any tubing or part must not come into prolonged contact with carbon monoxide. Nickel carbonyl decomposes readily back to Ni and CO upon contact with hot surfaces, and this method is used for the industrial purification of nickel in the Mond process.[55]
In nickel carbonyl and other carbonyls, the electron pair on the carbon interacts with the metal; the carbon monoxide donates the electron pair to the metal. In these situations, carbon monoxide is called the carbonyl ligand. One of the most important metal carbonyls is iron pentacarbonyl, Fe(CO)5:
Many metal–CO complexes are prepared by decarbonylation of organic solvents, not from CO. For instance, iridium trichloride and triphenylphosphine react in boiling 2-methoxyethanol or DMF to afford IrCl(CO)(PPh3)2.
Metal carbonyls in coordination chemistry are usually studied using
Organic and main group chemistry
In the presence of strong acids and water, carbon monoxide reacts with
Although CO reacts with carbocations and carbanions, it is relatively nonreactive toward organic compounds without the intervention of metal catalysts.[58]
With main group reagents, CO undergoes several noteworthy reactions.
·C
2O2−
2. It reacts with molten potassium to give a mixture of an organometallic compound, potassium acetylenediolate 2K+
·C
2O2−
2, potassium benzenehexolate 6K+
C
6O6−
6,[59] and potassium rhodizonate 2K+
·C
6O2−
6.[60]
The compounds cyclohexanehexone or triquinoyl (C6O6) and cyclopentanepentone or leuconic acid (C5O5), which so far have been obtained only in trace amounts, can be regarded as polymers of carbon monoxide. At pressures exceeding 5 GPa, carbon monoxide converts to polycarbonyl, a solid polymer that is metastable at atmospheric pressure but is explosive.[61][62]
Laboratory preparation
Carbon monoxide is conveniently produced in the laboratory by the dehydration of formic acid or oxalic acid, for example with concentrated sulfuric acid.[56][57][63] Another method is heating an intimate mixture of powdered zinc metal and calcium carbonate, which releases CO and leaves behind zinc oxide and calcium oxide:
- Zn + CaCO3 → ZnO + CaO + CO
Silver nitrate and iodoform also afford carbon monoxide:
- CHI3 + 3AgNO3 + H2O → 3HNO3 + CO + 3AgI
Finally, metal oxalate salts release CO upon heating, leaving a carbonate as byproduct:
- Na
2C
2O
4 → Na
2CO
3 + CO
Production
Thermal combustion is the most common source for carbon monoxide. Carbon monoxide is produced from the partial oxidation of carbon-containing compounds; it forms when there is not enough oxygen to produce carbon dioxide (CO2), such as when operating a stove or an internal combustion engine in an enclosed space.
A large quantity of CO byproduct is formed during the oxidative processes for the production of chemicals. For this reason, the process off-gases have to be purified.
Many methods have been developed for carbon monoxide production.[64]
Industrial production
A major industrial source of CO is producer gas, a mixture containing mostly carbon monoxide and nitrogen, formed by combustion of carbon in air at high temperature when there is an excess of carbon. In an oven, air is passed through a bed of coke. The initially produced CO2 equilibrates with the remaining hot carbon to give CO.[65] The reaction of CO2 with carbon to give CO is described as the Boudouard reaction.[66] Above 800 °C, CO is the predominant product:
- CO2 (g) + C (s) → 2 CO (g) (ΔHr = 170 kJ/mol)
Another source is "water gas", a mixture of hydrogen and carbon monoxide produced via the endothermic reaction of steam and carbon:
- H2O (g) + C (s) → H2 (g) + CO (g) (ΔHr = 131 kJ/mol)
Other similar "synthesis gases" can be obtained from natural gas and other fuels.
Carbon monoxide can also be produced by high-temperature electrolysis of carbon dioxide with solid oxide electrolyzer cells.[67] One method developed at DTU Energy uses a cerium oxide catalyst and does not have any issues of fouling of the catalyst.[68][69]
- 2 CO2 → 2 CO + O2
Carbon monoxide is also a byproduct of the reduction of metal oxide ores with carbon, shown in a simplified form as follows:
- MO + C → M + CO
Carbon monoxide is also produced by the direct oxidation of carbon in a limited supply of oxygen or air.
- 2 C + O2 → 2 CO
Since CO is a gas, the reduction process can be driven by heating, exploiting the positive (favorable) entropy of reaction. The Ellingham diagram shows that CO formation is favored over CO2 in high temperatures.
Use
Chemical industry
Carbon monoxide is an industrial gas that has many applications in bulk chemicals manufacturing.[70] Large quantities of aldehydes are produced by the hydroformylation reaction of alkenes, carbon monoxide, and H2. Hydroformylation is coupled to the Shell higher olefin process to give precursors to detergents.
- CO + Cl2 → COCl2
Methanol is produced by the hydrogenation of carbon monoxide. In a related reaction, the hydrogenation of carbon monoxide is coupled to C−C bond formation, as in the Fischer–Tropsch process where carbon monoxide is hydrogenated to liquid hydrocarbon fuels. This technology allows coal or biomass to be converted to diesel.
In the
Metallurgy
Carbon monoxide is a strong reductive agent and has been used in pyrometallurgy to reduce metals from ores since ancient times. Carbon monoxide strips oxygen off metal oxides, reducing them to pure metal in high temperatures, forming carbon dioxide in the process. Carbon monoxide is not usually supplied as is, in the gaseous phase, in the reactor, but rather it is formed in high temperature in presence of oxygen-carrying ore, or a carboniferous agent such as coke, and high temperature. The blast furnace process is a typical example of a process of reduction of metal from ore with carbon monoxide.
Likewise,
Lasers
Carbon monoxide has also been used as a lasing medium in high-powered infrared lasers.[72]
Proposed use as fuel on Mars
Carbon monoxide has been proposed for use as a fuel on Mars.
Biological and physiological properties
Physiology
Carbon monoxide is a bioactive molecule which acts as a gaseous signaling molecule. It is naturally produced by many enzymatic and non-enzymatic pathways,[74] the best understood of which is the catabolic action of heme oxygenase on the heme derived from hemoproteins such as hemoglobin.[75] Following the first report that carbon monoxide is a normal neurotransmitter in 1993,[8] carbon monoxide has received significant clinical attention as a biological regulator.
Because of carbon monoxide's role in the body, abnormalities in its metabolism have been linked to a variety of diseases, including neurodegenerations, hypertension, heart failure, and pathological inflammation.
Medicine
Studies involving carbon monoxide have been conducted in many laboratories throughout the world for its anti-inflammatory and cytoprotective properties.[79] These properties have the potential to be used to prevent the development of a series of pathological conditions including ischemia reperfusion injury, transplant rejection, atherosclerosis, severe sepsis, severe malaria, or autoimmunity.[78] Many pharmaceutical drug delivery initiatives have developed methods to safely administer carbon monoxide, and subsequent controlled clinical trials have evaluated the therapeutic effect of carbon monoxide.[80]
Microbiology
Microbiota may also utilize carbon monoxide as a gasotransmitter.[81] Carbon monoxide sensing is a signaling pathway facilitated by proteins such as CooA.[82][83][84] The scope of the biological roles for carbon monoxide sensing is still unknown.
The human microbiome produces, consumes, and responds to carbon monoxide.[74] For example, in certain bacteria, carbon monoxide is produced via the reduction of carbon dioxide by the enzyme carbon monoxide dehydrogenase with favorable bioenergetics to power downstream cellular operations.[85][74] In another example, carbon monoxide is a nutrient for methanogenic archaea which reduce it to methane using hydrogen.[86]
Carbon monoxide has certain antimicrobial properties which have been studied to treat against infectious diseases.[74]
Food science
Carbon monoxide is used in modified atmosphere packaging systems in the US, mainly with fresh meat products such as beef, pork, and fish to keep them looking fresh. The benefit is two-fold, carbon monoxide protects against microbial spoilage and it enhances the meat color for consumer appeal.[87] The carbon monoxide combines with myoglobin to form carboxymyoglobin, a bright-cherry-red pigment. Carboxymyoglobin is more stable than the oxygenated form of myoglobin, oxymyoglobin, which can become oxidized to the brown pigment metmyoglobin. This stable red color can persist much longer than in normally packaged meat. Typical levels of carbon monoxide used in the facilities that use this process are between 0.4% and 0.5%.[87]
The technology was first given "
Toxicity
Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries. For example Carbon monoxide poisoning leads to 50,000–100,000 emergency room visits and 1,500–2,000 deaths each year in the United States alone. Even with treatment, survivors often suffer from long-term cardiac and neurocognitive deficits, highlighting a clear unmet medical need for novel therapeutic strategies that reduce morbidity and mortality associated with CO poisoning. [92]
Weaponization
In ancient history,
Carbon monoxide had been used for
See also
- Breath carbon monoxide – Level of carbon monoxide in exhaled breath
- Carbon monoxide (data page) – Chemical data page
- Carbon monoxide detector – Device that measures carbon monoxide (CO)
- Criteria air pollutants– US EPA limits on certain air pollutants
- List of highly toxic gases
References
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0105". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Carbon monoxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ISBN 978-1-4377-7869-4. Retrieved 5 September 2015.
- ^ "Carbon Monoxide - CAMEO Chemicals". cameochemicals.noaa.gov. US NOAA Office of Response and Restoration.
- ^ ISBN 978-3527306732.
- ISBN 3527306730.
- ^ Voiland, Adam. "Fourteen years of carbon monoxide from MOPITT". Climate Change: Vital Signs of the Planet. Retrieved 2022-03-04.
- ^ S2CID 233205099.
- ^ Stromeyer, Friedrich (1808). Grundriß der theoretischen Chemie: zum Behuf seiner Vorlesungen entworfen (in German). Röwer. pp. 1–18.
- ISBN 9780128014578.
- .
- ISBN 978-1-43982077-3.
- ISBN 978-1-43982077-3.
- ^ Common Bond Energies (D) and Bond Lengths (r). wiredchemist.com
- ^ Vidal, C. R. (28 June 1997). "Highly Excited Triplet States of Carbon Monoxide". Archived from the original on 2006-08-28. Retrieved August 16, 2012.
- OCLC 46959719.
- )
- doi:10.1063/1.460293.
- S2CID 11905354.
- OCLC 476311784.
- .
- PMID 19275137.
- .
- S2CID 96291857.
- ^ Global Maps. Carbon Monoxide. earthobservatory.nasa.gov
- .
- ^ Source for figures: Carbon dioxide, NOAA Earth System Research Laboratory, (updated 2010.06). Methane, IPCC TAR table 6.1 Archived 2007-06-15 at the Wayback Machine, (updated to 1998). The NASA total was 17 ppmv over 100%, and CO2 was increased here by 15 ppmv. To normalize, N2 should be reduced by about 25 ppmv and O2 by about 7 ppmv.
- ISBN 978-0-309-02631-4.
- ^ Green W. "An Introduction to Indoor Air Quality: Carbon Monoxide (CO)". United States Environmental Protection Agency. Retrieved 2008-12-16.
- ^ Gosink, Tom (1983-01-28). "What Do Carbon Monoxide Levels Mean?". Alaska Science Forum. Geophysical Institute, University of Alaska Fairbanks. Archived from the original on 2008-12-25. Retrieved 2007-12-01.
- ISBN 978-9027704023.
- ^ a b "Carbon Monoxide Poisoning: Vehicles (AEN-208)". abe.iastate.edu. Retrieved 11 February 2018.
- ^ Gosink T (January 28, 1983). "What Do Carbon Monoxide Levels Mean?". Alaska Science Forum. Geophysical Institute, University of Alaska Fairbanks. Archived from the original on December 25, 2008. Retrieved December 16, 2008.
- S2CID 25223868.
- ISBN 978-0-471-72018-8.
- ISBN 978-1-84755-915-9.
- ISBN 978-0-444-01515-0.
- ^ Drummond, James (February 2, 2018). "MOPITT, Atmospheric Pollution, and Me: A Personal Story". Canadian Meteorological and Oceanographic Society. Retrieved August 1, 2018.
- .
- .
- .
- ISBN 978-0-309-02531-7.
- ^ a b Carbon Monoxide Questions and Answers from U.S. Consumer Product Safety Commission. Retrieved 2023-02-20. Archive copy at the Wayback Machine (archived 2023-01-17).
- ^ Treisman, Rachel (18 February 2021). "'A Disaster Within A Disaster': Carbon Monoxide Poisoning Cases Are Surging In Texas". NPR. Retrieved 2021-05-16.
- ^ "MSHA - Occupational Illness and Injury Prevention Program - Health Topics - Carbon Monoxide". arlweb.msha.gov. Archived from the original on 2017-12-31. Retrieved 2017-12-31.
- .
- S2CID 231639096.
- ^ Khan, Amina. "Did two planets around nearby star collide? Toxic gas holds hints". Los Angeles Times. Retrieved March 9, 2014.
- S2CID 206553853. Retrieved March 9, 2014.
- S2CID 58889896.
- Bibcode:1998A&A...330..375G.
- ^ Yeomans, Donald K. (2005). "Comets (World Book Online Reference Center 125580)". NASA. Archived from the original on 29 April 2005. Retrieved 18 August 2022.
About 80 percent of the ice is water ice, and frozen carbon monoxide makes up another 15 percent.
- S2CID 102343522.
- ISSN 2451-9294.
- .
- ^ a b Koch, H.; Haaf, W. (1973). "1-Adamantanecarboxylic Acid". Organic Syntheses; Collected Volumes, vol. 5, p. 20.
- ^ a b Coleman, G. H.; Craig, David (1943). "p-Tolualdehyde". Organic Syntheses; Collected Volumes, vol. 2, p. 583.
- .
- ^ Fownes, George (1869). A Manual of elementary chemistry. H.C. Lea. p. 678.
- .
- .
- ISBN 978-0121266011.
- ISBN 0-12-352651-5.
- ^ "Carbon Monoxide". Retrieved 21 May 2021.
- ISBN 978-0-7506-7707-3.
- PMID 28165079.
- ^ "New route to carbon-neutral fuels from carbon dioxide discovered by Stanford-DTU team - DTU". dtu.dk.
- S2CID 202640892– via www.nature.com.
- ISBN 978-3-527-28165-7.
- ISBN 978-3527306732.
- S2CID 112510554.
- doi:10.2514/2.3739.
- ^ S2CID 224824871.
- PMID 16601269.
- S2CID 17538129.
- S2CID 231762031.
- ^ S2CID 205477130.
- PMID 28077358.
- S2CID 51712930.
- PMID 30190328.
- PMID 15353565.
- S2CID 208217502.
- PMID 26021768.
- ISBN 978-3-527-30990-0.
- PMID 9782487.
- ^ PMID 28465017.
- PMID 22064057.
- ^ "Proof in the Pink? Meat Treated to Give It Fresh Look". ABC News. November 14, 2007. Retrieved May 5, 2009.
- ^ Carbon Monoxide in Meat Packaging: Myths and Facts. American Meat Institute. 2008. Archived from the original on 2011-07-14. Retrieved May 5, 2009.
- ^ "CO in packaged meat". Carbon Monoxide Kills Campaign. Archived from the original on September 26, 2010. Retrieved May 5, 2009.
- ISSN 0066-4219.
- ISBN 978-1-4051-0041-0.
External links
- Global map of carbon monoxide distribution
- Explanation of the structure
- International Chemical Safety Card 0023
- CDC NIOSH Pocket Guide to Chemical Hazards: Carbon monoxide—National Institute for Occupational Safety and Health (NIOSH), US Centers for Disease Control and Prevention (CDC)
- External MSDS data sheet
- Carbon Monoxide Detector Placement
- Microscale Gas Chemistry Experiments with Carbon Monoxide
- "Instant insight: Don't blame the messenger". Chemical Biology (11: Research News). 18 October 2007. Archived from the original on 28 October 2007. Retrieved 27 October 2019.
Outlining the physiology of carbon monoxide from the Royal Society of Chemistry.





