Carcinoma
| Carcinoma | |
|---|---|
red blood cells. Cytopathology specimen. Field stain. | |
| Specialty | Oncology |
Carcinoma is a malignancy that develops from
Carcinomas occur when the
Classification
As of 2004, no simple and comprehensive classification system has been devised and accepted within the scientific community.[5] Traditionally, however, malignancies have generally been classified into various types using a combination of criteria, including:[6]
The cell type from which they start; specifically:
- Epithelial cells ⇨ carcinoma
- Non-hematopoietic mesenchymal cells ⇨ sarcoma
- Hematopoieticcells
- Bone marrow–derived cells that normally mature in the bloodstream ⇨ leukemia
- Bone marrow-derived cells that normally mature in the lymphatic system ⇨ lymphoma
- Germ cells ⇨ germinoma
Other criteria that play a role include:
- The degree to which the malignant cells resemble their normal, untransformed counterparts
- The appearance of the local tissue and stromal architecture
- The anatomical location from which tumors arise
- Genetic, epigenetic, and molecular features
Histological types


- Adenocarcinoma (adeno = gland)
- Refers to a carcinoma featuring microscopic glandular-related tissue cytology, tissue architecture, and/or gland-related molecular products, e.g., mucin.
- Squamous cell carcinoma
- Refers to a carcinoma with observable features and characteristics indicative of squamous differentiation (intercellular bridges, keratinization, squamous pearls).
- Adenosquamous carcinoma
- Refers to a mixed tumor containing both adenocarcinoma and squamous cell carcinoma, wherein each of these cell types comprise at least 10% of the tumor volume.
- Anaplastic carcinoma
- Refers to a heterogeneous group of high-grade carcinomas that feature cells lacking distinct histological or cytological evidence of any of the more specifically differentiated anaplastic or undifferentiatedcarcinomas.
- Large cell carcinoma
- Composed of large, monotonous rounded or overtly polygonal-shaped cells with abundant cytoplasm.
- Small cell carcinoma
- Cells are usually round and are less than approximately three times the diameter of a resting lymphocyte and with little evident cytoplasm. Occasionally, small cell malignancies may themselves have significant components of slightly polygonal and/or spindle-shaped cells.[8]
There are a large number of rare subtypes of anaplastic, undifferentiated carcinoma. Some of the more well known include the lesions containing pseudo-
-
Adenosquamous carcinoma, with glandular features at left and squamous features at right.
-
Anaplastic tumor cells.
-
Large cell carcinoma.
-
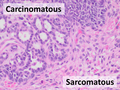
Carcinosarcoma, having mixed carcinomatous and sarcomatous elements
Carcinoma of unknown primary site
The term carcinoma has also come to encompass malignant tumors composed of transformed
ICD-10 code
- (8010-8045) neoplasms, NOS
- (8050-8080) neoplasms
- (M8070/3) Squamous cell carcinoma, NOS
- (M8070/3)
- (8090-8110) neoplasms
- (M8090/3) Basal cell carcinoma, NOS
- (M8090/3)
- (8120-8130) carcinomas
- (8140-8380) Adenocarcinomas
- (M8140/3) Adenocarcinoma, NOS
- (M8142/3) Linitis plastica
- (M8155/3) Vipoma
- (M8160/3) Cholangiocarcinoma
- (M8170/3) Hepatocellular carcinoma, NOS
- (M8200/3) Adenoid cystic carcinoma
- (M8312/3) Renal cell carcinoma
- (M8312/3) Grawitz tumor
- (8390-8420) Adnexal and Neoplasms
- (8430-8439) Neoplasms
- (8440-8490) Neoplasms
- (8500-8540) Ductal, Lobular and Neoplasms
- (8550-8559) neoplasms
- (8560-8580) neoplasms
Carcinoma In situ
The term carcinoma in situ (or CIS) is a term for cells that are significantly abnormal but not cancer.[9] They are thus not typically carcinomas.[10]
Pathogenesis
Cancer occurs when a single progenitor cell accumulates
- the ability to continue to divide perpetually, producing an exponentially (or near-exponentially) increasing number of new malignant cancerous "daughter cells" (uncontrolled mitosis);
- the ability to penetrate normal body surfaces and barriers, and to bore into or through nearby body structures and tissues (local invasiveness);
- the ability to spread to other sites within the body (blood vessels (distant metastasis).[11]
If this process of continuous growth, local invasion, and regional and distant metastasis is not halted via a combination of stimulation of immunological defenses and medical treatment interventions, the result is that the host has a continuously increasing burden of tumor cells throughout the body. Eventually, the tumor burden increasingly interferes with normal biochemical functions carried out by the host's
Carcinoma is but one form of cancer—one composed of cells that have developed the cytological appearance, histological architecture, or molecular characteristics of epithelial cells.
Invasion and metastasis
The hallmark of a
Mutation
Whole genome sequencing has established the mutation frequency for whole human genomes. The mutation frequency in the whole genome between generations for humans (parent to child) is about 70 new mutations per generation.[14]
Carcinomas, however, have much higher mutation frequencies. The particular frequency depends on tissue type, whether a mis-match DNA repair deficiency is present, and exposure to DNA damaging agents such as components of tobacco smoke. Tuna and Amos have summarized the mutation frequencies per megabase (Mb) in some carcinomas,[15] as shown in the table (along with the indicated frequencies of mutations per genome).
| Cell type | Mutation frequency | |
|---|---|---|
| Per megabase | Per diploid genome | |
| Germline | 0.023 | 70 |
| Prostate cancer | 0.9 | 5,400 |
| Colorectal carcinoma | ~5 | ~30,000 |
| Microsatellite stable (MSS) colon cancer | 2.8 | 16,800 |
| Microsatellite instable (MSI) colon cancer (mismatch repair deficient) | 47 | 282,000 |
| Hepatocellular carcinoma | 4.2 | 25,200 |
| Breast cancer | 1.18–1.66 | 7,080–9,960 |
| Lung cancer | 17.7 | 106,200 |
| Small cell lung cancer | 7.4 | 44,400 |
| Non-small cell lung cancer (smokers) | 10.5 | 63,000 |
| Non-small cell lung cancer (never-smokers) | 0.6 | 3,600 |
| Lung adenocarcinoma (smokers) | 9.8 | 58,500 |
| Lung adenocarcinoma (never-smokers) | 1.7 | 10,200 |
Cause of mutations
The likely major underlying cause of mutations in carcinomas is DNA damage.[
High frequency
The high frequency of mutations in the total genome within carcinomas suggests that, often, an early carcinogenic alteration may be a deficiency in DNA repair. For instance, mutation rates substantially increase (sometimes by 100-fold) in cells defective in DNA mismatch repair.[17]
A deficiency in DNA repair, itself, can allow DNA damages to accumulate, and error-prone translesion synthesis past some of those damages may give rise to mutations. In addition, faulty repair of these accumulated DNA damages may give rise to epigenetic alterations or epimutations. While a mutation or epimutation in a DNA repair gene, itself, would not confer a selective advantage, such a repair defect may be carried along as a passenger in a cell when the cell acquires an additional mutation/epimutation that does provide a proliferative advantage. Such cells, with both proliferative advantages and one or more DNA repair defects (causing a very high mutation rate), likely give rise to the high frequency of total genome mutations seen in carcinomas.
DNA repair
In somatic cells, deficiencies in DNA repair sometimes arise by mutations in DNA repair genes, but much more often are due to
Diagnosis
Carcinomas can be definitively diagnosed through
Types
- Oral: Most oral cancers are squamous-cell carcinoma
- Lung: Carcinoma comprises >98% of all lung cancers.
- Breast: Nearly all breast cancers are ductal carcinoma.
- Prostate: The most common form of carcinoma of the prostate is adenocarcinoma.
- Colon and rectum: Nearly all malignancies of the colon and rectum are either adenocarcinoma or squamous cell carcinoma.
- Pancreas: Pancreatic carcinoma is almost always of the adenocarcinoma type and is highly lethal.
- Ovaries: One of the most deadly forms due to late detection.[20]
Some carcinomas are named for their or the putative cell of origin, (e.g.hepatocellular carcinoma, renal cell carcinoma).
Staging
Staging of carcinoma refers to the process of combining physical/clinical examination, pathological review of cells and tissues, surgical techniques, laboratory tests, and imaging studies in a logical fashion to obtain information about the size of the neoplasm and the extent of its invasion and metastasis. Carcinoma stage is the variable that has been most consistently and tightly linked to the prognosis of the malignancy.
Carcinomas are usually staged with Roman numerals. In most classifications, Stage I and Stage II carcinomas are confirmed when the tumor has been found to be small and/or to have spread to local structures only. Stage III carcinomas typically have been found to have spread to regional lymph nodes, tissues, and/or organ structures, while Stage IV tumors have already metastasized through the blood to distant sites, tissues, or organs.
In some types of carcinomas, Stage 0 carcinoma has been used to describe carcinoma in situ, and occult carcinomas detectable only via examination of sputum for malignant cells (in lung carcinomas).
In more recent staging systems, substages (a, b, c) are becoming more commonly used to better define groups of patients with similar prognosis or treatment options.
The criteria for staging can differ dramatically based upon the organ system in which the tumor arises. For example, the colon[21] and bladder cancer[22] staging system relies on depth of invasion, staging of breast carcinoma is more dependent on the size of the tumor, and in renal carcinoma, staging is based on both the size of the tumor and the depth of the tumor invasion into the renal sinus. Carcinoma of the lung has a more complicated staging system, taking into account a number of size and anatomic variables.[23]
The UICC/AJCC
Grading
Grading of carcinomas refers to the employment of criteria intended to semi-quantify the degree of cellular and tissue maturity seen in the transformed cells relative to the appearance of the normal parent epithelial tissue from which the carcinoma derives.
Grading of carcinoma is most often done after a treating physician and/or surgeon obtains a sample of suspected tumor tissue using
, or other methods. Finally, the pathologist classifies the tumor semi-quantitatively into one of three or four grades, including:- Grade 1, or well differentiated: there is a close, or very close, resemblance to the normal parent tissue, and the tumor cells are easily identified and classified as a particular malignant histological entity;
- Grade 2, or moderately differentiated: there is considerable resemblance to the parent cells and tissues, but abnormalities can commonly be seen and the more complex features are not particularly well-formed;
- Grade 3, or poorly differentiated: there is very little resemblance between the malignant tissue and the normal parent tissue, abnormalities are evident, and the more complex architectural features are usually rudimentary or primitive;
- Grade 4, or undifferentiated carcinoma: these carcinomas bear no significant resemblance to the corresponding parent cells and tissues, with no visible formation of glands, ducts, bridges, stratified layers, keratin pearls, or other notable characteristics consistent with a more highly differentiated neoplasm.
Although there is definite and convincing statistical correlation between carcinoma grade and tumor prognosis for some tumor types and sites of origin, the strength of this association can be highly variable. It may be stated generally, however, that the higher the grade of the lesion, the worse is its prognosis.[25][26]
Epidemiology
While cancer is generally considered a disease of old age, children can also develop cancer.[27] In contrast to adults, carcinomas are exceptionally rare in children. Less than 1% of carcinoma diagnoses are in children.[28]
The two biggest risk factors for ovarian carcinoma are age and family history.[29]
References
- ISBN 9781841100500.
- )
- ^ "Definition of Carcinoma". Archived from the original on 10 October 2012. Retrieved 27 January 2014.
- ^ Oxford English Dictionary, 3rd edition, s.v.
- ^ PMID 15113444.
- ^ PMID 15571625.
- ^ Image by Mikael Häggström, MD. Source for findings: Caroline I.M. Underwood, M.D., Carolyn Glass, M.D., Ph.D. "Lung - Small cell carcinoma". Pathology Outlines.
{{cite web}}: CS1 maint: multiple names: authors list (link) Last author update: 20 September 2022 - ^ ISBN 978-92-832-2418-1. Archived from the original(PDF) on 23 August 2009. Retrieved 27 January 2014.
- ISBN 9780387310565.
- PMID 20541153.
- ^ "Carcinoma". Academic Press Dictionary of Science and Technology.
{{cite journal}}: Cite journal requires|journal=(help) - ^ PMID 28125050.
- PMID 30845172.
- PMID 20220176.
- PMID 23178448.
- PMID 21802474.
- PMID 16728433.
- PMID 15888787.
- ^ Wagman LD (2008). "Principles of Surgical Oncology". In Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (eds.). Cancer Management: A Multidisciplinary Approach (11th ed.). Archived from the original on 4 October 2013. Retrieved 8 June 2009.
- PMID 23849309.
- PMID 20524862.
- PMID 16526987.
- S2CID 24598745.
- ^ "What is Cancer Staging?". Archived from the original on 25 October 2007. Retrieved 27 January 2014.
- PMID 16678584.
- ^ "Poorly differentiated cancer from an unknown primary site". Retrieved 6 June 2022.
- PMID 11523714. Archived from the originalon 5 May 2010.
- ^ "Key Statistics for Childhood Cancers". www.cancer.org. Retrieved 6 May 2019.
- PMID 19817326.