Chemical structure
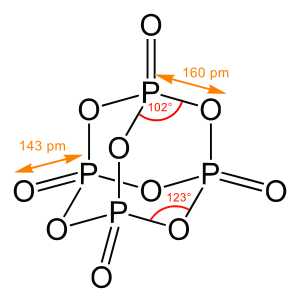
A chemical structure of a
).Background
Theories of chemical structure were first developed by
Concerning chemical structure, one has to distinguish between pure connectivity of the atoms within a molecule (chemical constitution), a description of a three-dimensional arrangement (molecular configuration, includes e.g. information on chirality) and the precise determination of bond lengths, angles and torsion angles, i.e. a full representation of the (relative) atomic coordinates.
In determining structures of chemical compounds, one generally aims to obtain, first and minimally, the pattern and degree of bonding between all atoms in the molecule; when possible, one seeks the three dimensional spatial coordinates of the atoms in the molecule (or other solid).[5]
Structural elucidation
The methods by which one can determine the structure of a molecule is called structural elucidation. These methods include:
- concerning only connectivity of the atoms: vibrational spectroscopies, infrared and Raman, provide, respectively, important supporting information about the numbers and adjacencies of multiple bonds, and about the types of functional groups (whose internal bonding gives vibrational signatures); further inferential studies that give insight into the contributing electronic structure of molecules include cyclic voltammetry and X-ray photoelectron spectroscopy.
- concerning precise metric three-dimensional information: can be obtained for gases by gas electron diffraction and microwave (rotational) spectroscopy (and other rotationally resolved spectroscopy) and for the crystalline solid state by X-ray crystallography[6] or neutron diffraction. These technique can produce three-dimensional models at atomic-scale resolution, typically to a precision of 0.001 Å for distances and 0.1° for angles (in unusual cases even better).[7][6]
Additional sources of information are: When a molecule has an unpaired electron spin in a
See also
- Structural chemistry
- Chemical structure diagram
- Crystallographic database
- MOGADOC A data base for experimental structures determined in the gas phase
- Pauli exclusion principle
- Chemical graph generator
References
Further reading
- Gallagher, Warren (2006). "Lecture 7: Structure Determination by X-ray Crystallography". Chem 406: Biophysical Chemistry (PDF) (self-published course notes). Eau Claire, WI, USA: University of Wisconsin-Eau Claire, Department of Chemistry. Retrieved July 2, 2014.
- Ward, S. C.; Lightfoot, M. P.; Bruno, I. J.; Groom, C. R. (April 1, 2016). "The Cambridge Structural Database". Acta Crystallographica Section B. 72 (2): 171–179. PMID 27048719.
