Chemotaxis
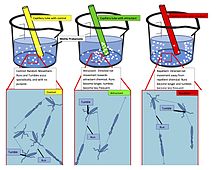
Chemotaxis (from
Positive chemotaxis occurs if the movement is toward a higher concentration of the chemical in question; negative chemotaxis if the movement is in the opposite direction. Chemically prompted kinesis (randomly directed or nondirectional) can be called chemokinesis.
History of chemotaxis research
Although migration of cells was detected from the early days of the development of microscopy by
Bacterial chemotaxis—general characteristics

Some bacteria, such as E. coli, have several flagella per cell (4–10 typically). These can rotate in two ways:
- Counter-clockwise rotation aligns the flagella into a single rotating bundle, causing the bacterium to swim in a straight line; and
- Clockwise rotation breaks the flagella bundle apart such that each flagellum points in a different direction, causing the bacterium to tumble in place.[15]
The directions of rotation are given for an observer outside the cell looking down the flagella toward the cell.[16]
Behavior
The overall movement of a bacterium is the result of alternating tumble and swim phases, called run-and-tumble motion.[17] As a result, the trajectory of a bacterium swimming in a uniform environment will form a random walk with relatively straight swims interrupted by random tumbles that reorient the bacterium.[18] Bacteria such as E. coli are unable to choose the direction in which they swim, and are unable to swim in a straight line for more than a few seconds due to rotational diffusion; in other words, bacteria "forget" the direction in which they are going. By repeatedly evaluating their course, and adjusting if they are moving in the wrong direction, bacteria can direct their random walk motion toward favorable locations.[19]
In the presence of a chemical gradient bacteria will chemotax, or direct their overall motion based on the gradient. If the bacterium senses that it is moving in the correct direction (toward attractant/away from repellent), it will keep swimming in a straight line for a longer time before tumbling; however, if it is moving in the wrong direction, it will tumble sooner. Bacteria like E. coli use temporal sensing to decide whether their situation is improving or not, and in this way, find the location with the highest concentration of attractant, detecting even small differences in concentration.[20]
This biased random walk is a result of simply choosing between two methods of random movement; namely tumbling and straight swimming.[21] The helical nature of the individual flagellar filament is critical for this movement to occur. The protein structure that makes up the flagellar filament, flagellin, is conserved among all flagellated bacteria.[22] Vertebrates seem to have taken advantage of this fact by possessing an immune receptor (TLR5) designed to recognize this conserved protein. [23]
As in many instances in biology, there are bacteria that do not follow this rule. Many bacteria, such as Vibrio, are monoflagellated and have a single flagellum at one pole of the cell. Their method of chemotaxis is different. Others possess a single flagellum that is kept inside the cell wall. These bacteria move by spinning the whole cell, which is shaped like a corkscrew.[24][page needed]
Signal transduction

Chemical gradients are sensed through multiple
Flagellum regulation
The proteins CheW and CheA bind to the receptor. The absence of receptor activation results in
Receptor regulation
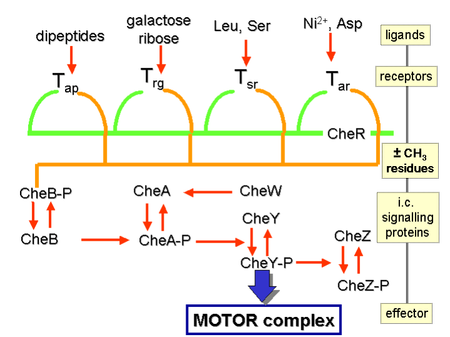
CheB, when activated by CheA, acts as a
Chemoattractants and chemorepellents
Chemoattractants and chemorepellents are
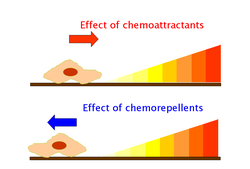
Effects of chemoattractants are elicited via chemoreceptors such as
Chemoattractants or chemorepellents bind MCPs at its extracellular domain; an intracellular signaling domain relays the changes in concentration of these chemotactic ligands to downstream proteins like that of CheA which then relays this signal to flagellar motors via phosphorylated CheY (CheY-P).[35] CheY-P can then control flagellar rotation influencing the direction of cell motility.[35]
For E.coli,
Chemoattractants in eukaryotes are well characterized for immune cells.
Mechanisms concerning chemorepellents are less known than chemoattractants. Although chemorepellents work to confer an avoidance response in organisms, Tetrahymena thermophila adapt to a chemorepellent, Netrin-1 peptide, within 10 minutes of exposure; however, exposure to chemorepellents such as GTP, PACAP-38, and nociceptin show no such adaptations.[38] GTP and ATP are chemorepellents in micro-molar concentrations to both Tetrahymena and Paramecium. These organisms avoid these molecules by producing avoiding reactions to re-orient themselves away from the gradient.[39]
Eukaryotic chemotaxis
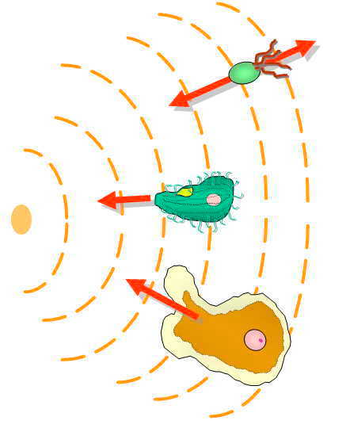
The mechanism of chemotaxis that
Eukaryotic cells are much larger than prokaryotes and have receptors embedded uniformly throughout the cell membrane.[42] Eukaryotic chemotaxis involves detecting a concentration gradient spatially by comparing the asymmetric activation of these receptors at the different ends of the cell.[42] Activation of these receptors results in migration towards chemoattractants, or away from chemorepellants.[42] In mating yeast, which are non-motile, patches of polarity proteins on the cell cortex can relocate in a chemotactic fashion up pheromone gradients.[44][8]
It has also been shown that both prokaryotic and eukaryotic cells are capable of chemotactic memory.
Levels of receptors, intracellular signalling pathways and the effector mechanisms all represent diverse, eukaryotic-type components. In eukaryotic unicellular cells, amoeboid movement and cilium or the eukaryotic flagellum are the main effectors (e.g.,
Motility
Unlike motility in bacterial chemotaxis, the mechanism by which eukaryotic cells physically move is unclear. There appear to be mechanisms by which an external chemotactic gradient is sensed and turned into an intracellular

Chemotaxis refers to the directional migration of cells in response to chemical gradients; several variations of chemical-induced migration exist as listed below.
- Chemokinesis refers to an increase in cellular motility in response to chemicals in the surrounding environment. Unlike chemotaxis, the migration stimulated by chemokinesis lacks directionality, and instead increases environmental scanning behaviors.[53]
- In ligands is responsible for induction of transendothelial migration and angiogenesis.
- Necrotaxis embodies a special type of chemotaxis when the chemoattractant molecules are released from necrotic or apoptotic cells. Depending on the chemical character of released substances, necrotaxis can accumulate or repel cells, which underlines the pathophysiological significance of this phenomenon.
Receptors
In general, eukaryotic cells sense the presence of chemotactic stimuli through the use of 7-transmembrane (or serpentine) heterotrimeric
The main classes of chemotaxis receptors are triggered by:- Formyl peptides - formyl peptide receptors (FPR),
- Chemokines - chemokine receptors(CCR or CXCR), and
- Leukotrienes - leukotriene receptors (BLT).[58]
However, induction of a wide set of membrane receptors (e.g.,
Chemotactic selection

While some chemotaxis receptors are expressed in the surface membrane with long-term characteristics, as they are determined genetically, others have short-term dynamics, as they are assembled ad hoc in the presence of the ligand.[60] The diverse features of the chemotaxis receptors and ligands allows for the possibility of selecting chemotactic responder cells with a simple chemotaxis assay By chemotactic selection, we can determine whether a still-uncharacterized molecule acts via the long- or the short-term receptor pathway.[61] The term chemotactic selection is also used to designate a technique that separates eukaryotic or prokaryotic cells according to their chemotactic responsiveness to selector ligands.[62][non-primary source needed][non-primary source needed]
Chemotactic ligands


The number of molecules capable of eliciting chemotactic responses is relatively high, and we can distinguish primary and secondary chemotactic molecules.[citation needed] The main groups of the primary ligands are as follows:
- Formyl peptides are di-, tri-, tetrapeptides of bacterial origin, formylated on the N-terminus of the peptide.[Formyl peptide receptors.
- Complement 3a (C3a) and complement 5a (C5a) are intermediate products of the complement cascade.[citation needed] Their synthesis is joined to the three alternative pathways (classical, lectin-dependent, and alternative) of complement activation by a convertase enzyme.[citation needed] The main target cells of these derivatives are neutrophil granulocytes and monocytes as well.[citation needed]
- RANTES), and CXC chemokines are neutrophil granulocyte-specific (e.g., IL-8).[citation needed] Investigations of the three-dimensional structures of chemokines provided evidence that a characteristic composition of beta-sheets and an alpha helix provides expression of sequences required for interaction with the chemokine receptors.[citation needed] Formation of dimers and their increased biological activity was demonstrated by crystallography of several chemokines, e.g. IL-8.[citation needed]
- Metabolites of polyunsaturated fatty acids
- The family of bacterialinfections. After leaving nearby blood vessels, these cells recognize chemicals produced by bacteria in a cut or scratch and migrate "toward the smell".
- 5-hydroxyeicosatrienoic acid and 5-oxoeicosatrienoic acid are metabolites of Mead acid (5Z,8Z,11Z-eicosatrirenoid acid); they stimulate leukocyte chemotaxis through the oxoeicosanoid receptor 1[65] with 5-oxoeicosatrienoic acid being as potent as its arachidonic acid-derived analog, 5-oxo-eicosatetraenoic acid, in stimulating human blood eosinophil and neutrophil chemotaxis.[64]
- 12-Hydroxyeicosatetraenoic acid is an eicosanoid metabolite of arachidonic acid made by ALOX12 which stimulates leukocyte chemotaxis through the leukotriene B4 receptor, BLT2.[64]
- cyclooxygenase 2 that stimulates chemotaxis through the Prostaglandin DP2 receptor. It elicits chemotactic responses in eosinophils, basophils, and T helper cells of the Th2 subtype.[66][non-primary source needed][non-primary source needed]
- 12-Hydroxyheptadecatrienoic acid is a non-eicosanoid metabolite of arachidonic acid made by cyclooxygenase 1 or cyclooxygenase 2 that stimulates leukocyte chemotaxis though the leukotriene B4 receptor, BLT2.[67][non-primary source needed][non-primary source needed]
- 15-oxo-eicosatetraenoic acid is an eicosanoid metabolite of arachidonic acid made my ALOX15; it has weak chemotactic activity for human monocytes (sees 15-Hydroxyeicosatetraenoic acid#15-oxo-ETE).[68][non-primary source needed][non-primary source needed] The receptor or other mechanism by which this metabolite stimulates chemotaxis has not been elucidated.
Chemotactic range fitting
Chemotactic responses elicited by
Clinical significance
A changed migratory potential of cells has relatively high importance in the development of several clinical symptoms and syndromes. Altered chemotactic activity of extracellular (e.g., Escherichia coli) or intracellular (e.g., Listeria monocytogenes) pathogens itself represents a significant clinical target. Modification of endogenous chemotactic ability of these microorganisms by pharmaceutical agents can decrease or inhibit the ratio of infections or spreading of infectious diseases. Apart from infections, there are some other diseases wherein impaired chemotaxis is the primary etiological factor, as in Chédiak–Higashi syndrome, where giant intracellular vesicles inhibit normal migration of cells.
| Type of disease | Chemotaxis increased | Chemotaxis decreased |
|---|---|---|
| Infections | Inflammations | AIDS, Brucellosis
|
| Chemotaxis results in the disease | — | Kartagener syndrome
|
| Chemotaxis is affected | metastatic tumors |
Hodgkin disease, male infertility
|
| Intoxications | benzpyrene |
Hg and Cr salts, ozone |
Mathematical models
Several mathematical models of chemotaxis were developed depending on the type of
- Migration (e.g., basic differences of bacterial swimming, movement of unicellular eukaryotes with amoeboidmigration)
- Physico-chemical characteristics of the chemicals (e.g., diffusion) working as ligands
- Biological characteristics of the ligands (attractant, neutral, and repellent molecules)
- Assay systems applied to evaluate chemotaxis (see incubation times, development, and stability of concentration gradients)
- Other environmental effects possessing direct or indirect influence on the migration (lighting, temperature, magnetic fields, etc.)
Although interactions of the factors listed above make the behavior of the solutions of mathematical models of chemotaxis rather complex, it is possible to describe the basic phenomenon of chemotaxis-driven motion in a straightforward way. Indeed, let us denote with the spatially non-uniform concentration of the chemo-attractant and as its gradient. Then the chemotactic cellular flow (also called current) that is generated by the chemotaxis is linked to the above gradient by the law:[70]
where is the spatial density of the cells and is the so-called 'Chemotactic coefficient' - is often not constant, but a decreasing function of the chemo-attractant. For some quantity that is subject to total flux and generation/destruction term , it is possible to formulate a continuity equation:
where is the divergence. This general equation applies to both the cell density and the chemo-attractant. Therefore, incorporating a diffusion flux into the total flux term, the interactions between these quantities are governed by a set of coupled reaction-diffusion partial differential equations describing the change in and :[70]
where describes the growth in cell density, is the kinetics/source term for the chemo-attractant, and the diffusion coefficients for cell density and the chemo-attractant are respectively and .
Spatial ecology of soil microorganisms is a function of their chemotactic sensitivities towards substrate and fellow organisms.[71][non-primary source needed][non-primary source needed] The chemotactic behavior of the bacteria was proven to lead to non-trivial population patterns even in the absence of environmental heterogeneities. The presence of structural pore scale heterogeneities has an extra impact on the emerging bacterial patterns.
Measurement of chemotaxis
A wide range of techniques is available to evaluate chemotactic activity of cells or the chemoattractant and chemorepellent character of ligands. The basic requirements of the measurement are as follows:
- Concentration gradients can develop relatively quickly and persist for a long time in the system
- Chemotactic and chemokinetic activities are distinguished
- Migration of cells is free toward and away on the axis of the concentration gradient
- Detected responses are the results of active migration of cells
Despite the fact that an ideal chemotaxis assay is still not available, there are several protocols and pieces of equipment that offer good correspondence with the conditions described above. The most commonly used are summarised in the table below:
| Type of assay | Agar-plate assays | Two-chamber assays | Others |
|---|---|---|---|
| Examples |
|
|
|
Artificial chemotactic systems
Chemical robots that use artificial chemotaxis to navigate autonomously have been designed.[72][73] Applications include targeted delivery of drugs in the body.[74] More recently, enzyme molecules have also shown positive chemotactic behavior in the gradient of their substrates.[75] The thermodynamically-favorable binding of enzymes to their specific substrates is recognized as the origin of enzymatic chemotaxis.[76] Additionally, enzymes in cascades have also shown substrate-driven chemotactic aggregation.[77]
Apart from active enzymes, non-reacting molecules also show chemotactic behavior. This has been demonstrated by using dye molecules that move directionally in gradients of polymer solution through favorable hydrophobic interactions.[78]
See also
References
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 6 (11th ed.). Cambridge University Press. p. 77.
- PMID 27231052.
- PMID 29546880.
- PMID 25301842.
- PMID 29042028.
- PMID 26271598.
- S2CID 53116963.
- ^ PMID 34050026.
- ^ Chemotaxis Lecture. Uploaded in 2007. available at: http://www.rpgroup.caltech.edu/courses/aph161/2007/lectures/ChemotaxisLecture.pdf Archived 2010-06-19 at the Wayback Machine (Last inspected: 15/04/17)
- ^ Élie Metchnikoff". Encyclopædia Britannica. Encyclopædia Britannica, Inc.
- ^ Keller-Segel Models for Chemotaxis. 2012. available at: http://www.isn.ucsd.edu/courses/Beng221/problems/2012/BENG221_Project%20-%20Roberts%20Chung%20Yu%20Li.pdf Archived 29 August 2017 at the Wayback Machine (Last inspected by April 2017)
- PMID 4873021.
- S2CID 7221477.
- S2CID 35733036.
- PMID 20615986.
- ^ "Bacterial Chemotaxis" (PDF). Archived (PDF) from the original on 6 May 2017.
- S2CID 1909173.
- PMID 22169400.
- ISBN 978-0-691-00064-0.
- PMID 22169400.
- PMID 4560688.
- PMID 34299141.
- PMID 28845044.
- ISBN 978-0-387-00888-2.[page needed]
- S2CID 205493118.
- PMID 15955239.
- ISBN 978-0-19-960848-5.
- ^ PMID 9442881.
- ^ ToxCafe (2 June 2011). "Chemotaxis". Archived from the original on 11 July 2015. Retrieved 23 March 2017 – via YouTube.
- ^ S2CID 205493118.
- ISBN 9789812707932.
- S2CID 5334523.
- PMID 15539117.
- .
- ^ PMID 15187186.
- ^ PMID 2403544.
- ^ PMID 1093163.
- PMID 27123011.
- PMID 18404496.
- ISBN 978-3-319-32211-7
- PMID 911982.
- ^ PMID 24363460.
- ^ S2CID 207440927.
- PMID 32186970.
- ^ PMID 25249632.
- S2CID 4660870.
- PMID 20864631.
- PMID 18685153.
- S2CID 248703226.
- S2CID 31704677.
- S2CID 11331182.
- PMID 26820523.
- PMID 199132.
- S2CID 4212997.
- PMID 8807851.
- S2CID 14193715.
- PMID 26928542.
- PMID 29873835.
- S2CID 27784085.
- ISBN 978-3-319-32211-7.[page needed]
- ISBN 978-3-319-32211-7.
- S2CID 33755476.
- PMID 264125.
- ^ PMID 25449650.
- PMID 24056189.
- PMID 17767353.
- PMID 25480980.
- S2CID 23499393.
- PMID 14995080.
- ^ ISBN 978-0-387-95223-9. Archived(PDF) from the original on 6 May 2022.
- .
- S2CID 257388244. Retrieved 13 March 2023.
- S2CID 243966350.
- S2CID 84150518.
- PMID 23308365.
- S2CID 52816076.
- PMID 29461522.
- PMID 29064685.
Further reading
- Alberts B, Johnson A, Lewis J, Walter P, Raff MC (2002). "Bacterial Chemotaxis Depends on a Two-Component Signaling Pathway Activated by Histidine-Kinase-associated Receptors". Molecular Biology of the Cell. Taylor & Francis Group. ISBN 978-0-8153-4069-0. Retrieved 18 September 2017.
- Bagorda A, Parent CA (August 2008). "Eukaryotic chemotaxis at a glance". Journal of Cell Science. 121 (Pt 16): 2621–4. PMID 18685153.
- Berg HC (1993). Random walks in biology (Expanded, rev. ed.). Princeton, NJ: Princeton Univ. Press. ISBN 978-0-691-00064-0.
- Berg HC (2003). E. coli in motion. Vol. 58. New York: Springer. pp. 64–65. )
- Dusenbery DB (2009). Living at micro scale : the unexpected physics of being small. Cambridge, Mass.: Harvard University Press. ISBN 978-0-674-03116-6.
- Eisenbach M (2004). Lengeler JW (ed.). Chemotaxis. London: Imperial College Press. ISBN 978-1-86094-413-0.
- Eisenbach, Michael (December 2011). "Bacterial Chemotaxis". Encyclopedia of Life Sciences. )
- Hazelbauer GL (13 October 2012). "Bacterial chemotaxis: the early years of molecular studies". Annual Review of Microbiology. 66 (1): 285–303. PMID 22994495.
- Jin T, Hereld D (2016). Chemotaxis: Methods and Protocols. Humana Press. ISBN 978-1-4939-3480-5.
- Miller LD, Russell MH, Alexandre G (2009). Diversity in bacterial chemotactic responses and niche adaptation. Vol. 66. pp. 53–75. )
- Rao CV, Kirby JR, Arkin AP (February 2004). "Design and diversity in bacterial chemotaxis: a comparative study in Escherichia coli and Bacillus subtilis". PLOS Biology. 2 (2): E49. PMID 14966542.
- Williams AH (20 December 2010). "Chemotaxis on the move - active learning teaching tool". Journal of Microbiology & Biology Education. 11 (2): 177–8. PMID 23653726.











![{\displaystyle {\begin{aligned}{\partial C \over {\partial t}}&=f(C)+\nabla \cdot \left[D_{C}\nabla C-C\chi (\varphi )\nabla \varphi \right]\\{\partial \varphi \over {\partial t}}&=g(\varphi ,C)+\nabla \cdot (D_{\varphi }\nabla \varphi )\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/924b3d46d7dc2a27228a7d98cf43d035a4622c41)



