Chloride
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Chloride[1] | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| 3587171 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| 14910 | |||
IUPHAR/BPS |
|||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| Cl− | |||
| Molar mass | 35.45 g·mol−1 | ||
Conjugate acid
|
Hydrogen chloride | ||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
153.36 J·K−1·mol−1[2] | ||
Std enthalpy of (ΔfH⦵298)formation |
−167 kJ·mol−1[2] | ||
| Related compounds | |||
Other anions
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
The term chloride refers to a compound or molecule that contains either a chlorine ion (Cl−), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (−Cl). Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is /ˈklɔːraɪd/.[3]
The chlorine ion is an
The chloride is also a neutral chlorine atom covalently bonded by a single bond to the rest of the molecule. For example, methyl chloride CH3Cl is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Other examples of covalent chlorides are carbon tetrachloride CCl4, sulfuryl chloride SO2Cl2 and monochloramine NH2Cl.
Electronic properties
A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm). The chlorine atom's hold on the valence shell is weaker because the chloride anion has one more electron than it does.[5] The ion is colorless and diamagnetic. In aqueous solution, it is highly soluble in most cases; however, for some chloride salts, such as silver chloride, lead(II) chloride, and mercury(I) chloride, they are only slightly soluble in water.[6] In aqueous solution, chloride is bonded by the protic end of the water molecules.
Reactions of chloride
Chloride can be oxidized but not reduced. The first oxidation, as employed in the chlor-alkali process, is conversion to chlorine gas. Chlorine can be further oxidized to other oxides and oxyanions including hypochlorite (ClO−, the active ingredient in chlorine bleach), chlorine dioxide (ClO2), chlorate (ClO−
3), and perchlorate (ClO−
4).
In terms of its acid–base properties, chloride is a
- NaCl + H2SO4 → NaHSO4 + HCl
Ionic chloride salts react with other salts to exchange anions. The presence of halide ions like chloride can be detected using silver nitrate. A solution containing chloride ions will produce a white silver chloride precipitate:[7]
- Cl− + Ag+ → AgCl
The concentration of chloride in an assay can be determined using a chloridometer, which detects silver ions once all chloride in the assay has precipitated via this reaction.
Chlorided silver electrodes are commonly used in ex vivo electrophysiology.[8]
Other oxyanions
Chlorine can assume oxidation states of −1, +1, +3, +5, or +7. Several neutral chlorine oxides are also known.
Chlorine oxidation state −1 +1 +3 +5 +7 Name chloride hypochlorite chlorite chlorate perchlorate Formula Cl− ClO− ClO−
2ClO−
3ClO−
4Structure 




Occurrence in nature
In nature, chloride is found primarily in seawater, which has a chloride ion concentration of 19400 mg/liter. Most chloride salts are soluble in water, thus, chloride-containing minerals are usually only found in abundance in dry climates or deep underground. Some chloride-containing minerals include
Role in biology
Chloride has a major physiological significance, which includes regulation of
Chloride is an essential
Chloride is usually (though not always) at a higher extracellular concentration, causing it to have a negative reversal potential (around −61 mV at 37 °C in a mammalian cell).[15] Characteristic concentrations of chloride in model organisms are: in both E. coli and budding yeast are 10–200 mM (dependent on medium), in mammalian cells 5–100 mM and in blood plasma 100 mM.[16]
The concentration of chloride in the blood is called
Corrosion

The presence of chlorides, such as in seawater, significantly worsens the conditions for pitting corrosion of most metals (including stainless steels, aluminum and high-alloyed materials). Chloride-induced corrosion of steel in concrete lead to a local breakdown of the protective oxide form in alkaline concrete, so that a subsequent localized corrosion attack takes place.[19]
Environmental threats
Increased concentrations of chloride can cause a number of ecological effects in both aquatic and terrestrial environments. It may contribute to the acidification of streams, mobilize radioactive soil metals by ion exchange, affect the mortality and reproduction of aquatic plants and animals, promote the invasion of saltwater organisms into previously freshwater environments, and interfere with the natural mixing of lakes. Sodium chloride has also been shown to change the composition of microbial species at relatively low concentrations. It can also hinder the denitrification process, a microbial process essential to nitrate removal and the conservation of water quality, and inhibit the nitrification and respiration of organic matter.[20]
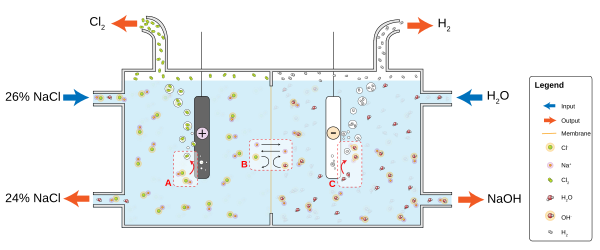
Production
The
- 2 Cl− → Cl
2 + 2 e− - 2 H
2O + 2 e− → H2 + 2 OH−
Examples and uses
An example is table salt, which is
Calcium chloride (CaCl2) is a salt that is marketed in
Examples of
Water quality and processing
A major application involving chloride is desalination, which involves the energy intensive removal of chloride salts to give potable water. In the petroleum industry, the chlorides are a closely monitored constituent of the mud system. An increase of the chlorides in the mud system may be an indication of drilling into a high-pressure saltwater formation. Its increase can also indicate the poor quality of a target sand.[citation needed]
Chloride is also a useful and reliable chemical indicator of river and groundwater fecal contamination, as chloride is a non-reactive solute and ubiquitous to sewage and potable water. Many water regulating companies around the world utilize chloride to check the contamination levels of the rivers and potable water sources.[22]
Food
Chloride salts such as sodium chloride are used to preserve food and as nutrients or condiments.
See also
- Halide (compounds of halogens)
- Renal chloride reabsorption
References
- ^ "Chloride ion - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ ISBN 978-0-618-94690-7.
- ISBN 9781405881180
- ^ a b Green, John, and Sadru Damji. "Chapter 3." Chemistry. Camberwell, Vic.: IBID, 2001. Print.
- ^ "Size of Atoms". chemed.chem.purdue.edu. Retrieved 2022-03-03.
- ISBN 978-1-285-13370-6.
- ^ "Testing for halide ions - Group 0 and testing ions - GCSE Chemistry (Single Science) Revision - WJEC". BBC Bitesize. Retrieved 2022-03-03.
- ISBN 978-0-471-48685-5.
- ^ "Chloride and Salinity" (PDF). colombia.edu. 8 September 2011. Retrieved 8 January 2023.
- ISBN 9780750628327.
- PMID 14907900.
- PMID 22385875.
- PMID 30539650.
- PMID 11917096.
- ^ "Equilibrium potentials". www.d.umn.edu.
- ^ Milo, Ron; Philips, Rob. "Cell Biology by the Numbers: What are the concentrations of different ions in cells?". book.bionumbers.org. Retrieved 24 March 2017.
- PMID 27267918.
- PMID 31082167.
- ISBN 978-1-78242-276-1.
- ISBN 978-0-12-370626-3.
- ^ "Common Salts". hyperphysics.phy-astr.gsu.edu. Georgia State University.
- ^ "Chlorides". www.gopetsamerica.com. Archived from the original on 18 August 2016. Retrieved 14 April 2018.





