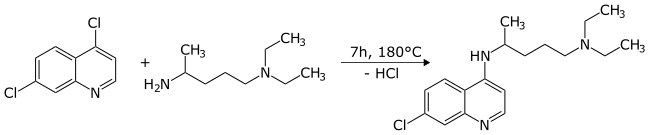
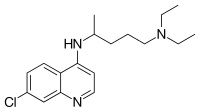
Chloroquine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈklɔːrəkwiːn/ |
| Trade names | Aralen, other |
| Other names | Chloroquine phosphate |
| AHFS/Drugs.com | Monograph |
| License data |
|
By mouth | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 1-2 months |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Chloroquine is a medication primarily used to prevent and treat
Common side effects include muscle problems, loss of appetite, diarrhea, and skin rash.
Chloroquine was discovered in 1934 by
Medical uses
Malaria

♦ Elevated occurrence of chloroquine- or multi-resistant malaria
♦ Occurrence of chloroquine-resistant malaria
♦ No Plasmodium falciparum or chloroquine-resistance
♦ No malaria
Chloroquine has been used in the treatment and prevention of malaria from Plasmodium vivax, P. ovale, and P. malariae. It is generally not used for Plasmodium falciparum as there is widespread resistance to it.[9][10]
Chloroquine has been extensively used in
Amebiasis
In treatment of amoebic liver abscess, chloroquine may be used instead of or in addition to other medications in the event of failure of improvement with metronidazole or another nitroimidazole within five days or intolerance to metronidazole or a nitroimidazole.[13]
Rheumatic disease
As it mildly suppresses the
Side effects
Side effects include blurred vision, nausea, vomiting, abdominal cramps, headache, diarrhea, swelling legs/ankles, shortness of breath, pale lips/nails/skin, muscle weakness, easy bruising/bleeding, hearing and mental problems.[14][15]
- Unwanted/uncontrolled movements (including tongue and face twitching,
- Deafness or tinnitus[14]
- Nausea, vomiting, diarrhea, abdominal cramps[15]
- Headache[14]
- Mental/mood changes (such as confusion, personality changes, unusual thoughts/behavior, depression, feeling being watched, hallucinating)[14][15]
- Signs of serious infection (such as high fever, severe chills, persistent sore throat)[14]
- Skin itchiness, skin color changes, hair loss, and skin rashes[15][17]
- Chloroquine-induced itching is very common among black Africans (70%), but much less common in other races. It increases with age, and is so severe as to stop compliance with drug therapy. It is increased during malaria fever; its severity is correlated to the malaria parasite load in blood. Some evidence indicates it has a genetic basis and is related to chloroquine action with opiate receptors centrally or peripherally.[18]
- Triggering of a severe psoriasis attack in those with psoriasis[16]
- Unpleasant metallic taste
- This could be avoided by "taste-masked and controlled release" formulations such as multiple emulsions.[19]
- Chloroquine retinopathy (irreversible retinal damage)[16]
- Electrocardiographic changes[20]
- This manifests itself as either conduction disturbances (bundle-branch block, atrioventricular block) or cardiomyopathy — often with hypertrophy, restrictive physiology, and congestive heart failure. The changes may be irreversible. Only two cases have been reported requiring heart transplantation, suggesting this particular risk is very low. Electron microscopy of cardiac biopsies show pathognomonic cytoplasmic inclusion bodies.
- Pancytopenia, aplastic anemia, reversible agranulocytosis, low blood platelets, neutropenia[16]
- Worsening of the condition for those with porphyria[16]
Pregnancy
Chloroquine has not been shown to have any harmful effects on the fetus when used in the recommended doses for malarial prophylaxis.[21] Small amounts of chloroquine are excreted in the breast milk of lactating women. However, this drug can be safely prescribed to infants, the effects are not harmful. Studies with mice show that radioactively tagged chloroquine passed through the placenta rapidly and accumulated in the fetal eyes which remained present five months after the drug was cleared from the rest of the body.[16][22] Women who are pregnant or planning on getting pregnant are still advised against traveling to malaria-risk regions.[21]
Elderly
There is not enough evidence to determine whether chloroquine is safe to be given to people aged 65 and older. Since it is cleared by the kidneys, toxicity should be monitored carefully in people with poor kidney functions, as is more likely to be the case in the elderly.[16]
Drug interactions
Chloroquine has a number of
- Ampicillin – levels may be reduced by chloroquine;[16]
- Antacids – may reduce absorption of chloroquine;[16]
- Cimetidine – may inhibit metabolism of chloroquine; increasing levels of chloroquine in the body;[16]
- Cyclosporine – levels may be increased by chloroquine;[16]and
- Mefloquine – may increase risk of convulsions.[16]
Overdose
Chloroquine, in overdose, has a risk of death of about 20%.
While the usual dose of chloroquine used in treatment is 10 mg/kg, toxicity begins to occur at 20 mg/kg, and death may occur at 30 mg/kg.[23] In children as little as a single tablet can be fatal.[24][16]
Treatment recommendations include early
Pharmacology
This section needs additional citations for verification. (March 2024) |
Absorption of chloroquine is rapid and primarily happens in the gastrointestinal tract.[25] It is widely distributed in body tissues.[26] Protein binding in plasma ranges from 46% to 79%.[27] Its metabolism is partially hepatic, giving rise to its main metabolite, desethylchloroquine.[28] Its excretion is ≥50% as unchanged drug in urine, where acidification of urine increases its elimination.[citation needed] It has a very high volume of distribution, as it diffuses into the body's adipose tissue.[citation needed]
Accumulation of the drug may result in deposits that can lead to blurred vision and
Chloroquine is also a lysosomotropic agent, meaning it accumulates preferentially in the
Mechanism of action

Malaria
The lysosomotropic character of chloroquine is believed to account for much of its antimalarial activity; the drug concentrates in the acidic food vacuole of the parasite and interferes with essential processes. Its lysosomotropic properties further allow for its use for in vitro experiments pertaining to intracellular lipid related diseases,[30][31] autophagy, and apoptosis.[32]
Inside
Hemoglobin is composed of a protein unit (digested by the parasite) and a heme unit (not used by the parasite). During this process, the parasite releases the toxic and soluble molecule heme. The heme moiety consists of a porphyrin ring called Fe(II)-protoporphyrin IX (FP). To avoid destruction by this molecule, the parasite biocrystallizes heme to form hemozoin, a nontoxic molecule. Hemozoin collects in the digestive vacuole as insoluble crystals.[citation needed]
Chloroquine enters the red blood cell by simple diffusion, inhibiting the parasite cell and digestive vacuole. Chloroquine (CQ) then becomes protonated (to CQ2+), as the digestive vacuole is known to be acidic (pH 4.7); chloroquine then cannot leave by diffusion. Chloroquine caps hemozoin molecules to prevent further biocrystallization of heme, thus leading to heme buildup. Chloroquine binds to heme (or FP) to form the FP-chloroquine complex; this complex is highly toxic to the cell and disrupts membrane function. Action of the toxic FP-chloroquine and FP results in cell lysis and ultimately parasite cell autodigestion.[33] Parasites that do not form hemozoin are therefore resistant to chloroquine.[34]
Resistance in malaria
Since the first documentation of P. falciparum chloroquine resistance in the 1950s, resistant strains have appeared throughout East and West Africa, Southeast Asia, and South America. The effectiveness of chloroquine against P. falciparum has declined as resistant strains of the parasite evolved.
Resistant parasites are able to rapidly remove chloroquine from the digestive vacuole using a transmembrane pump. Chloroquine-resistant parasites pump chloroquine out at 40 times the rate of chloroquine-sensitive parasites; the pump is coded by the P. falciparum chloroquine resistance transporter (PfCRT) gene.[35] The natural function of the chloroquine pump is to transport peptides: mutations to the pump that allow it to pump chloroquine out impairs its function as a peptide pump and comes at a cost to the parasite, making it less fit.[36]
Resistant parasites also frequently have mutation in the ABC transporter P. falciparum multidrug resistance (PfMDR1) gene, although these mutations are thought to be of secondary importance compared to PfCRT. An altered chloroquine-transporter protein, CG2 has been associated with chloroquine resistance, but other mechanisms of resistance also appear to be involved.[37]
As of 2014[update] chloroquine is still effective against
Antiviral
Chloroquine has
Chloroquine also seems to act as a zinc ionophore that allows extracellular zinc to enter the cell and inhibit viral RNA-dependent RNA polymerase.[44][45]
Other
Chloroquine inhibits
Against
History
In Peru, the indigenous people extracted the bark of the Cinchona tree (Cinchona officinalis)[47] and used the extract to fight chills and fever in the seventeenth century. In 1633, this herbal medicine was introduced in Europe, where it was given the same use and also began to be used against malaria. The quinoline antimalarial drug quinine was isolated from the extract in 1820.[48]: 130–131
After World War I, the German government sought alternatives to quinine. Chloroquine, a synthetic analogue with the same
Chemical synthesis
The first synthesis of chloroquine was disclosed in a patent filed by
By 1949, chloroquine manufacturing processes had been established to allow its widespread use.[55]
Society and culture

Formulations
Chloroquine comes in tablet form as the phosphate, sulfate, and hydrochloride salts. Chloroquine is usually dispensed as the phosphate.[56]
Names
Brand names include Chloroquine FNA, Resochin, Dawaquin, and Lariago.[57]
Other animals
Chloroquine, in various chemical forms, is used to treat and control surface growth of anemones and algae, and many protozoan infections in aquariums,
Research
Chloroquine was proposed as a treatment for
Chloroquine was being considered in 2003, in pre-clinical models as a potential agent against chikungunya fever.[63]
COVID-19

Chloroquine and
Cleavage of the SARS-CoV-2 S2 spike protein required for viral entry into cells can be accomplished by proteases TMPRSS2 located on the cell membrane, or by cathepsins (primarily cathepsin L) in endolysosomes.[75] Hydroxychloroquine inhibits the action of cathepsin L in endolysosomes, but because cathepsin L cleavage is minor compared to TMPRSS2 cleavage, hydroxychloroquine does little to inhibit SARS-CoV-2 infection.[75]
Several countries initially used chloroquine or hydroxychloroquine for treatment of persons hospitalized with COVID-19 (as of March 2020), though the drug was not formally approved through clinical trials.
Their use was withdrawn as a possible treatment for COVID-19 infection when it proved to have no benefit for hospitalized patients with severe COVID-19 illness in the international Solidarity trial and UK RECOVERY Trial.[81][82] On 15 June 2020, the FDA revoked its emergency use authorization, stating that it was "no longer reasonable to believe" that the drug was effective against COVID-19 or that its benefits outweighed "known and potential risks".[83][84][85] In fall of 2020, the National Institutes of Health issued treatment guidelines recommending against the use of hydroxychloroquine for COVID-19 except as part of a clinical trial.[64]
In 2021, hydroxychloroquine was part of the recommended treatment for mild cases in India.[86]
In 2020, the speculative use of hydroxychloroquine for COVID-19 threatened its availability for people with established indications (malaria and auto-immune diseases).[68]Other
The
References
- ^ a b c d e f g h i j k l "Aralen Phosphate". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ "Chloroquine Use During Pregnancy". Drugs.com. Archived from the original on 16 April 2019. Retrieved 16 April 2019.
There are no controlled data in human pregnancies.
- ^ "Chloroquine or Hydroxychloroquine". COVID-19 Treatment Guidelines. National Institutes of Health. Archived from the original on 28 August 2020. Retrieved 14 February 2021.
- ISBN 978-1-4557-0737-9. Retrieved 25 March 2020.
- ISBN 978-1-4160-4470-3. Archivedfrom the original on 2 November 2018. Retrieved 9 September 2017.
- ISBN 978-3-319-40746-3. Archivedfrom the original on 1 November 2018. Retrieved 9 September 2017.
- hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06.
- ^ "Frequently Asked Questions (FAQs): If I get malaria, will I have it for the rest of my life?". US Centers for Disease Control and Prevention. 8 February 2010. Archived from the original on 13 May 2012. Retrieved 14 May 2012.
- PMID 16265887.
- PMID 16265886.
- ^ "Chloroquine phosphate tablet – chloroquine phosphate tablet, coated". dailymed.nlm.nih.gov. Archived from the original on 8 December 2015. Retrieved 4 November 2015.
- ^ CDC. Health information for international travel 2001–2002. Atlanta, Georgia: U.S. Department of Health and Human Services, Public Health Service, 2001.
- ^ Amebic Hepatic Abscesses~treatment at eMedicine
- ^ a b c d e f "Drugs & Medications". www.webmd.com. Retrieved 22 March 2020.
- ^ a b c d "Chloroquine Side Effects: Common, Severe, Long Term". Drugs.com. Retrieved 22 March 2020.
- ^ a b c d e f g h i j k l m "Chloroquine phosphate tablet". DailyMed. 8 October 2018. Retrieved 7 April 2020.
- ^ "Chloroquine: MedlinePlus Drug Information". medlineplus.gov. Retrieved 22 March 2020.
- PMID 11014416.
- PMID 7884629.
- S2CID 37926477.
- ^ a b "Malaria – Chapter 3 – 2016 Yellow Book". wwwnc.cdc.gov. Archived from the original on 14 January 2016. Retrieved 11 November 2015.
- S2CID 4191322.
- ^ S2CID 41205035.
- ^ PMID 15837026.
- ^ "Chloroquine". §6.1 Absorption by route of exposure. Retrieved 24 April 2020.
- S2CID 35269419.
- PMID 6849768.
- S2CID 2115928.
- S2CID 228089310.
- PMID 21711726.
- PMID 20427654.
- PMID 20308316.
- S2CID 30446678.
- (PDF) from the original on 22 September 2017. Retrieved 4 November 2018.
- S2CID 206520905.
- PMID 32764664.
- ^ Tripathi KD (2003). Essentials of Medical Pharmacology (fifth ed.). Jaypee Brothers Medical Publisher Ltd. pp. 739–740.
- PMID 23541983.
- ^ ISBN 9781119371199.
- PMID 10773485.
- PMID 14592603.
- PMID 28596841.
- PMID 12388705.
- PMID 25271834.
- PMID 21079686.
- PMID 23209439.
- ^ Fern K (2010–2020). "Cinchona officinalis – L." Plans for a Future. Archived from the original on 25 August 2017. Retrieved 2 February 2020.
- ^ PMID 25009879.
- ^ Kouznetsov VV, Amado Torres DF (September 2008). "Antimalarials: construction of molecular hybrids based on chloroquine". Universitas Scientiarum. 13 (3): 306–320.
- S2CID 54526057.
- ISBN 0471899801.
- S2CID 32186437.
- ^ "The History of Malaria, an Ancient Disease". Centers for Disease Control. 29 July 2019. Archived from the original on 28 August 2010.
- ^ DE patent 683692, Andersag, Hans; Breitner, Stefan & Jung, Heinrich, "Process for the preparation of quinoline compounds containing amino groups with basic substituents in the 4-position", issued 1939-11-13, assigned to IG Farbenindustrie AG
- .
- ^ "Chloroquine". nih.gov. National Institutes of Health. Retrieved 24 March 2020.
- ^ "Ipca Laboratories: Formulations – Branded". Archived from the original on 6 April 2019. Retrieved 14 March 2020.
- ^ Hemdal J (20 February 2013). "Aquarium Fish: Chloroquine: A "New" Drug for Treating Fish Diseases". Advanced Aquarist. Archived from the original on 15 March 2013. Retrieved 26 March 2020.
- ^ Francis-Floyd R, Floyd MR. "Amyloodinium ocellatum, an Important Parasite of Cultured Marine Fish" (PDF). agrilife.org. Archived from the original (PDF) on 1 June 2015. Retrieved 24 March 2020.
- ^ PMID 15351731.
- PMID 32171740.
- PMID 16115318.
- PMID 14592603.
- ^ a b "Chloroquine or Hydroxychloroquine". COVID-19 Treatment Guidelines. National Institutes of Health. Archived from the original on 28 August 2020. Retrieved 14 February 2021.
- ^ "Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020". FDA. 30 March 2020. Archived from the original on 19 October 2020. Retrieved 28 February 2021.
- PMID 33476807.
- PMID 32350928.
- ^ PMID 32269021.
- ^ "Assessment of Evidence for COVID-19-Related Treatments: Updated 4/3/2020". American Society of Health-System Pharmacists. Archived from the original on 14 April 2021. Retrieved 7 April 2020.
- PMID 32232419.
- PMID 33624299.
- ^ PMID 38425958.
- PMID 32438018.
- S2CID 266754199.
- ^ PMID 34611326.
- ^ "Information for clinicians on therapeutic options for COVID-19 patients". US Centers for Disease Control and Prevention. 21 March 2020. Archived from the original on 8 April 2020. Retrieved 22 March 2020.
- ^ Hinton DM (28 March 2020). "Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original on 2 October 2020. Retrieved 30 March 2020.
- ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Archived from the original on 8 April 2020. Retrieved 9 April 2020.
- PMID 32208486.
- ^ "FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems". U.S. Food and Drug Administration (FDA). 24 April 2020. Archived from the original on 4 November 2020. Retrieved 28 February 2021.
- ^ Mulier T (17 June 2020). "Hydroxychloroquine halted in WHO-sponsored COVID-19 trials". Bloomberg. Archived from the original on 11 October 2020. Retrieved 17 June 2020.
- ^ "No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19". Recovery Trial, Nuffield Department of Population Health, University of Oxford, UK. 5 June 2020. Archived from the original on 8 October 2020. Retrieved 7 June 2020.
- ^ "Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Archived from the original on 15 June 2020. Retrieved 15 June 2020.
- ^ Lovelace Jr B (15 June 2020). "FDA revokes emergency use of hydroxychloroquine". CNBC. Archived from the original on 11 October 2020. Retrieved 28 February 2021.
- ^ "Frequently Asked Questions on the Revocation of the Emergency Use Authorization for Hydroxychloroquine Sulfate and Chloroquine Phosphate" (PDF). U.S. Food and Drug Administration (FDA). 15 June 2020. Archived from the original on 15 April 2021. Retrieved 15 June 2020.
- ^ "Clinical Management Protocol for Covid-19 (in Adults)" (PDF). Ministry of Health and Family Welfare. 24 May 2021. Archived (PDF) from the original on 5 December 2021. Retrieved 10 July 2021. "Health ministry issues revised clinical management protocols for Covid-19 amid spurt in cases". Times of India. Press Trust of India. 13 June 2021. Archived from the original on 11 July 2021. Retrieved 10 July 2021.
- PMID 17012039.
- PMID 16520470.
- PMID 31855356.
External links
- "Medicines for the Prevention of Malaria While Traveling – Chloroquine (Aralen)" (PDF) (Fact sheet). U.S. Centers for Disease Control and Prevention (CDC).
 The dictionary definition of chloroquine at Wiktionary
The dictionary definition of chloroquine at Wiktionary