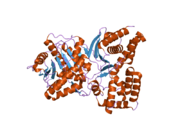Choline acetyltransferase
| Choline acetyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| CHAT | |||
|---|---|---|---|
Gene ontology | |||
| Molecular function | |||
| Cellular component | |||
| Biological process | |||
| Sources:Amigo / QuickGO | |||
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) |
| ||||||||
| RefSeq (protein) |
| ||||||||
| Location (UCSC) | Chr 10: 49.61 – 49.67 Mb | Chr 14: 32.13 – 32.19 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
Choline acetyltransferase (commonly abbreviated as ChAT, but sometimes CAT) is a
History
Choline acetyltransferase was first described by
An enzyme has been extracted from brain and nervous tissue which forms acetylcholine. The formation occurs only in presence of adenosinetriphosphate (ATP). The enzyme is called choline acetylase.
— Nachmanson & Machado, 1943[6]
The
Structure
The 3D structure of ChAT has been solved by X-ray crystallography
The choline substrate fits into a pocket in the interior of ChAT, while acetyl-CoA fits into a pocket on the surface of the protein. The 3D crystal structure shows the acetyl group of acetyl-CoA abuts the choline binding pocket – minimizing the distance between acetyl-group donor and receiver.
Homology
ChAT is very conserved across the animal genome. Among mammals, in particular, there is very high sequence similarity. Human and cat (Felis catus) ChAT, for example, have 89% sequence identity. Sequence identity with Drosophila is about 30%.[13]
Forms of ChAT
There are two forms of ChAT: Soluble form and membrane-bound form.[14] The soluble form accounts for 80-90% of the total enzyme activity while the membrane-bound form is responsible for the rest of 10-20% activity.[15] However, there has long been a debate on how the latter form of ChAT is bound to the membrane.[16] The membrane-bound form of ChAT is associated with synaptic vesicles.[17]
Common and peripheral isoforms of ChAT
There exist two isoforms of ChAT, both encoded by the same sequence. The common type ChAT (cChAT) is present in both the CNS and PNS. Peripheral type ChAT (pChAT) is preferentially expressed in the PNS in humans, and arises from
Function

It is often used as an immunohistochemical marker for motor neurons (motoneurons).
Mutations
Mutants of ChAT have been isolated in several species, including C. elegans, Drosophila, and humans. Most non-lethal mutants that have a non-wild type phenotype exhibit some activity, but significantly less than wild type.
In C. elegans, several mutations in ChAT have been traced to the cha-1 gene. All mutations result in a significant drop in ChAT activity. Percent activity loss can be greater than 98% in some cases. Phenotypic effects include slowed growth, decreased size, uncoordinated behavior, and lack of sensitivity toward
The human gene responsible for encoding ChAT is CHAT. Mutations in CHAT have been linked to congenital myasthenic syndrome, a disease which leads to general motor function deficiency and weakness. Further symptoms include fatal apnea. Out of ten isolated mutants, 1 has been shown to lack activity completely, 8 have been shown to have significantly decreased activity, and 1 has an unknown function.[21]
Clinical significance
Alzheimer's disease
The
Amyotrophic lateral sclerosis
The
Drugs
Neostigmine methylsulfate, an anticholinesterase agent, has been used to target ChAT. In particular, use of neostigmine methylsulfate has been shown to have positive effects against congenital myasthenic syndrome.[26]
Exposure to estradiol has been shown to increase ChAT in female rats.[27]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000070748 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021919 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 1840566.
- ^ .
- PMID 13115440.
- .
- PMID 21066687.
- PMID 20276121.
- PMID 5716187.
- PMID 15477102.
- S2CID 23621617.
- S2CID 44586742.
- S2CID 24196247.
- S2CID 4336737.
- S2CID 1139292.
- ^ Rand, James. "Acetylcholine". WormBook.
- PMID 6698395.
- S2CID 45897606.
- PMID 11172068.
- PMID 7046051.
- PMID 9600198.
- S2CID 23763400.
- S2CID 28937351.
- S2CID 8672902.
- S2CID 1525252.
Further reading
- Oda Y (2000). "Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system". Pathol. Int. 49 (11): 921–37. S2CID 23621617.
- Wang J, Fu X, Zhang D, Yu L, Li N, Lu Z, Gao Y, Wang M, Liu X, Zhou C, Han W, Yan B, Wang J (2017). "ChAT-positive neurons participate in subventricular zone neurogenesis after middle cerebral artery occlusion in mice". Behav. Brain Res. 316: 145–151. PMID 27609645.
- Oda Y, Nakanishi I, Deguchi T (1993). "A complementary DNA for human choline acetyltransferase induces two forms of enzyme with different molecular weights in cultured cells". Brain Res. Mol. Brain Res. 16 (3–4): 287–94. PMID 1337937.
- Wang J, Lu Z, Fu X, Zhang D, Yu L, Li N, Gao Y, Liu X, Yin C, Ke J, Li L, Zhai M, Wu S, Fan J, Lv L, Liu J, Chen X, Yang Q, Wang J (2017). "Alpha-7 Nicotinic Receptor Signaling Pathway Participates in the Neurogenesis Induced by ChAT-Positive Neurons in the Subventricular Zone". Transl Stroke Res. 8 (5): 484–493. PMID 28551702.
- Toussaint JL, Geoffroy V, Schmitt M, Werner A, Garnier JM, Simoni P, Kempf J (1992). "Human choline acetyltransferase (CHAT): partial gene sequence and potential control regions". Genomics. 12 (2): 412–6. PMID 1339386.
- Lorenzi MV, Trinidad AC, Zhang R, Strauss WL (1992). "Two mRNAs are transcribed from the human gene for choline acetyltransferase". DNA Cell Biol. 11 (8): 593–603. PMID 1388731.
- Misawa H, Ishii K, Deguchi T (1992). "Gene expression of mouse choline acetyltransferase. Alternative splicing and identification of a highly active promoter region". J. Biol. Chem. 267 (28): 20392–9. PMID 1400357.
- Cervini R, Rocchi M, DiDonato S, Finocchiaro G (1992). "Isolation and sub-chromosomal localization of a DNA fragment of the human choline acetyltransferase gene". Neurosci. Lett. 132 (2): 191–4. S2CID 23030200.
- Strauss WL, Kemper RR, Jayakar P, Kong CF, Hersh LB, Hilt DC, Rabin M (1991). "Human choline acetyltransferase gene maps to region 10q11-q22.2 by in situ hybridization". Genomics. 9 (2): 396–8. PMID 1840566.
- Viegas-Péquignot E, Berrard S, Brice A, Apiou F, Mallet J (1991). "Localization of a 900-bp-long fragment of the human choline acetyltransferase gene to 10q11.2 by nonradioactive in situ hybridization". Genomics. 9 (1): 210–2. PMID 2004764.
- Itoh N, Slemmon JR, Hawke DH, Williamson R, Morita E, Itakura K, Roberts E, Shively JE, Crawford GD, Salvaterra PM (1986). "Cloning of Drosophila choline acetyltransferase cDNA". Proc. Natl. Acad. Sci. U.S.A. 83 (11): 4081–5. PMID 3086876.
- Hersh LB, Takane K, Gylys K, Moomaw C, Slaughter C (1988). "Conservation of amino acid sequences between human and porcine choline acetyltransferase". J. Neurochem. 51 (6): 1843–5. S2CID 24613238.
- Berrard S, Brice A, Lottspeich F, Braun A, Barde YA, Mallet J (1988). "cDNA cloning and complete sequence of porcine choline acetyltransferase: in vitro translation of the corresponding RNA yields an active protein". Proc. Natl. Acad. Sci. U.S.A. 84 (24): 9280–4. PMID 3480542.
- Chireux MA, Le Van Thai A, Weber MJ (1995). "Human choline acetyltransferase gene: localization of alternative first exons". J. Neurosci. Res. 40 (4): 427–38. S2CID 42856768.
- Bausero P, Schmitt M, Toussaint JL, Simoni P, Geoffroy V, Queuche D, Duclaud S, Kempf J, Quirin-Stricker C (1993). "Identification and analysis of the human choline acetyltransferase gene promoter". NeuroReport. 4 (3): 287–90. PMID 7682855.
- Quirin-Stricker C, Nappey V, Simoni P, Toussaint JL, Schmitt M (1994). "Trans-activation by thyroid hormone receptors of the 5' flanking region of the human ChAT gene". Brain Res. Mol. Brain Res. 23 (3): 253–65. PMID 8057782.
- Erickson JD, Varoqui H, Schäfer MK, Modi W, Diebler MF, Weihe E, Rand J, Eiden LE, Bonner TI, Usdin TB (1994). "Functional identification of a vesicular acetylcholine transporter and its expression from a "cholinergic" gene locus". J. Biol. Chem. 269 (35): 21929–32. PMID 8071310.
- Kengaku M, Misawa H, Deguchi T (1993). "Multiple mRNA species of choline acetyltransferase from rat spinal cord". Brain Res. Mol. Brain Res. 18 (1–2): 71–6. PMID 8479291.
- Misawa H, Matsuura J, Oda Y, Takahashi R, Deguchi T (1997). "Human choline acetyltransferase mRNAs with different 5'-region produce a 69-kDa major translation product". Brain Res. Mol. Brain Res. 44 (2): 323–33. PMID 9073174.
- Lönnerberg P, Ibáñez CF (1999). "Novel, testis-specific mRNA transcripts encoding N-terminally truncated choline acetyltransferase". Mol. Reprod. Dev. 53 (3): 274–81. S2CID 39464614.
- Sakakibara A, Hattori S (2000). "Chat, a Cas/HEF1-associated adaptor protein that integrates multiple signaling pathways". J. Biol. Chem. 275 (9): 6404–10. PMID 10692442.
External links
- Choline+Acetyltransferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)