Chromium(III) chloride
 Anhydrous
| |
 Hexahydrate
| |
| Names | |
|---|---|
IUPAC name
| |
| Other names
Chromic chloride
| |
| Identifiers | |
| |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
ECHA InfoCard
|
100.030.023 |
| 1890 130477 532690 | |
PubChem CID
|
|
RTECS number
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CrCl3 | |
| Molar mass | 158.36 g/mol (anhydrous) 266.45 g/mol (hexahydrate)[1] |
| Appearance | Purple (anhydrous), dark green (hexahydrate) |
| Density | 2.87 g/cm3 (anhydrous) 1.760 g/cm3 (hexahydrate) |
| Melting point | 1,152 °C (2,106 °F; 1,425 K) (anhydrous) 81 °C (hexahydrate)[2] |
| Boiling point | 1,300 °C (2,370 °F; 1,570 K) decomposes |
| slightly soluble (anhydrous) 585 g/L (hexahydrate) | |
| Solubility | insoluble in ethanol insoluble in ether, acetone |
| Acidity (pKa) | 2.4 (0.2M solution) |
| +6890.0·10−6 cm3/mol | |
| Structure | |
YCl3 structure
| |
| Octahedral | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H314, H411 | |
| P260, P264, P270, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1870 mg/kg (oral, rat)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3[3] |
REL (Recommended)
|
TWA 0.5 mg/m3[3] |
IDLH (Immediate danger) |
250 mg/m3[3] |
| Safety data sheet (SDS) | ICSC 1316 (anhydrous) ICSC 1532 (hexahydrate) |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chromium(III) chloride (also called chromic chloride) is an
Structure
Anhydrous chromium(III) chloride adopts the
-
Space-filling model of cubic close packing of chloride ions in the crystal structure of CrCl3
-
Ball-and-stick model of part of a layer
-
Stacking of layers
Chromium(III) chloride hydrates
The hydrated chromium(III) chlorides display the somewhat unusual property of existing in a number of distinct chemical forms (isomers), which differ in terms of the number of chloride anions that are coordinated to Cr(III) and the water of crystallization. The different forms exist both as solids and in aqueous solutions. Several members are known of the series of [CrCl3−q(H2O)n]q+. The common hexahydrate can be more precisely described as [CrCl2(H2O)4]Cl·2H2O. It consists of the cation trans-[CrCl2(H2O)4]+ and additional molecules of water and a chloride anion in the lattice.[10] Two other hydrates are known, pale green [CrCl(H2O)5]Cl2·H2O and violet [Cr(H2O)6]Cl3. Similar hydration isomerism is seen with other chromium(III) compounds.
Preparation
Anhydrous chromium(III) chloride may be prepared by
- Cr2O3 + 3 C + 3 Cl2 → 2 CrCl3 + 3 CO
The hydrated chlorides are prepared by treatment of chromate with hydrochloric acid and aqueous methanol.
Reactions
Slow reaction rates are common with chromium(III) complexes. The low reactivity of the d3 Cr3+ ion can be explained using crystal field theory. One way of opening CrCl3 up to substitution in solution is to reduce even a trace amount to CrCl2, for example using zinc in hydrochloric acid. This chromium(II) compound undergoes substitution easily, and it can exchange electrons with CrCl3 via a chloride bridge, allowing all of the CrCl3 to react quickly. With the presence of some chromium(II), solid CrCl3 dissolves rapidly in water. Similarly, ligand substitution reactions of solutions of [CrCl2(H2O)4]+ are accelerated by chromium(II) catalysts.
With molten alkali metal chlorides such as potassium chloride, CrCl3 gives salts of the type M3[CrCl6] and K3[Cr2Cl9], which is also octahedral but where the two chromiums are linked via three chloride bridges.
The hexahydrate can also be dehydrated with thionyl chloride:[13]
- CrCl3·6H2O + 6 SOCl2 → CrCl3 + 6 SO2 + 12 HCl
Complexes with organic ligands
CrCl3 is a
- CrCl3 + 3 C5H5N → CrCl3(C5H5N)3
Treatment with
- CrCl3·6H2O + 12 (CH3)3SiCl → CrCl3(THF)3 + 6 ((CH3)3Si)2O + 12 HCl
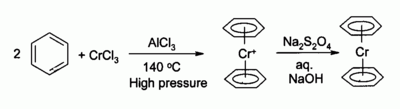
Precursor to organochromium complexes
Chromium(III) chloride is used as the precursor to many
Phosphine complexes derived from CrCl3 catalyse the trimerization of
Use in organic synthesis
One niche use of CrCl3 in
Chromium(III) chloride has also been used as a Lewis acid in organic reactions, for example to catalyse the nitroso
Dyestuffs
A number of chromium-containing dyes are used commercially for wool. Typical dyes are triarylmethanes consisting of ortho-hydroxylbenzoic acid derivatives.[18]
Precautions
Although
References
- ^ "Chromium(III) chloride sublimation, 99 10025-73-7".
- ^ "Chromium(III) chloride hexahydrate Technipur™ | Sigma-Aldrich". Retrieved 2022-08-16.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Chromium(III) compounds [as Cr(III)]". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Cameo Chemicals MSDS
- ^ ISSN 0957-4484.
- ISBN 978-0-08-037941-8.
- ^ A. F. Wells, Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984.
- S2CID 247452267.
- ^ D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- ISBN 978-0-32316129-9. Retrieved 2014-01-10.
- ISBN 9780470132401.
- ISBN 9780470132609.
- PMID 15228301.
Further reading
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- K. Takai, in Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, (R. M. Coates, S. E. Denmark, eds.), pp. 206–211, Wiley, New York, 1999.