Ciliopathy
| Ciliopathy | |
|---|---|
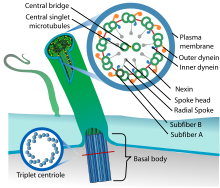 | |
| Eukaryotic cilium | |
| Specialty | Medical genetics |
A ciliopathy is any
Although ciliopathies are usually considered to involve proteins that localize to motile and/or immotile (primary)
Significant advances in understanding the importance of cilia were made in the mid-1990s. For example, the discovery of the role of cilia in embryonic development, identification of ciliary defects in genetic disorders such as Polycystic kidney disease, Bardet–Biedl syndrome and Primary ciliary dyskinesia.[5][6] However, the physiological role that this organelle plays in most tissues remains elusive. Additional studies of how ciliary dysfunction can lead to such severe disease and developmental pathologies is still a subject of current research.[7]
Signs and symptoms
A wide variety of symptoms are potential clinical features of ciliopathy. The signs most exclusive to a ciliopathy, in descending order of exclusivity, are:[8]: 138
- )
- Agenesis of the corpus callosum
- Situs inversus
- Posterior encephalocele
- Polycystic kidneys
- Postaxial polydactyly
- Liver disease
- Retinitis pigmentosa
- Intellectual disability
A case with polycystic ovary syndrome, multiple subcutaneous cysts, renal function impairment, Caroli disease and liver cirrhosis due to ciliopathy has been described.[9]
Phenotypes sometimes associated with ciliopathies can include:[8]
- Anencephaly
- Breathing abnormalities
- Cerebellar vermis hypoplasia
- Diabetes
- Exencephaly
- Eye movement abnormalities
- Hydrocephalus
- Hypoplasia of the corpus callosum
- Hypotonia
- Infertility
- Cognitive impairment/defects
- Obesity[10]
- Other polydactyly
- Respiratory dysfunction
- Renal cystic disease
- Retinal degeneration
- Sensorineural deafness
- Spina bifida
Pathophysiology
"In effect, the
In addition to this sensory role mediating specific signaling cues, cilia play "a
Signal transduction pathways involved include the Hedgehog signaling pathway and the Wnt signaling pathway.[12]
Dysfunctional cilia can lead to:
- Chemosensation abnormalities,[13] typically via ciliated epithelial cellular dysfunction.[1]
- Defective
- Cellular motility dysfunction[13]
- Issues with displacement of extracellular fluid[13]
In organisms of normal health, cilia are critical for:[15]
Genetics
"Just as different genes can contribute to similar diseases, so the same genes and families of genes can play a part in a range of different diseases." For example, in just two of the diseases caused by malfunctioning cilia, Meckel–Gruber syndrome and Bardet–Biedl syndrome, patients who carry mutations in genes associated with both diseases "have unique symptoms that are not seen in either condition alone." The genes linked to the two different conditions "interact with each other during development." Systems biologists are endeavoring to define functional modules containing multiple genes and then look at disorders whose phenotypes fit into such modules.[16]
A particular
Additionally, clinical presentations of patients with identical mutation can differ, suggesting the role of genetic modifiers.[17]
As of 2017, 187 ciliopathy associated genes have been confirmed, while the roles of further 241 candidate genes are still being investigated.[18]
A common way to identify ciliopathies such as ADPKD and ARPKD which have known genetic causes, is through linkage analysis direct mutation screening.[19] Other techniques, such as gene panels and whole-exome sequencing and whole genome sequencing can also be used to provide distinct advantages.[19][20] Gene panels analyse specific sets of genes and can be more comprehensive than single gene or direct mutation screening. Whole-exome/genome sequencing can screen for heterozygous carriers, and detect novel/rare variations.[19][21]
Mutations in the PKD1 and PKD2 genes which encode for polycystin-1 and polycistin-2 respectively are known to be causes of ADPKD, a ciliopathy that presents with the formation and growth of cysts in the kidneys, leading to renal failure.[22]
List of ciliopathies
"The phenotypic parameters that define a ciliopathy may be used to both recognize the cellular basis of a number of genetic disorders and to facilitate the diagnosis and treatment of some diseases of unknown" cause.[8]
Known ciliopathies
Likely ciliopathies
| Condition | OMIM
|
Gene(s) | Systems/organs affected |
|---|---|---|---|
| Acrocallosal syndrome[23] | 200990 | KIF7, GLI3 | |
Acromelic frontonasal dysostosis[23]
|
603671 | ZSWIM6 | |
| Arima syndrome[23] | 243910 | ||
| Biemond syndrome[23] | 113400 | ||
| COACH syndrome[23] | 216360 | TMEM67, CC2D2A, RPGRIP1L | |
| Conorenal syndrome[27][23] | 266920 | ||
| Greig cephalopolysyndactyly syndrome[23] | 175700 | GLI3 | |
| Hydrolethalus syndrome[23] | 236680 | HYLS1 | |
| Johanson–Blizzard syndrome[23] | 243800 | UBR1 | |
oral-facial-digital syndrome type 2)[23]
|
252100 | ||
| Neu–Laxova syndrome[23] | 256520 | ||
| Opitz G/BBB syndrome[23] | 300000 | MID1 | |
| Pallister–Hall syndrome[23] | 146510 | GLI3 | |
| Papillorenal syndrome[23] | 120330 | PAX2 | |
| Renal–hepatic–pancreatic dysplasia[23] | 208540 | NPHP3 | |
Varadi–Papp syndrome (oral-facial-digital syndrome type 6)[23]
|
277170 |
Possible ciliopathies
History
In 1674-1677, the Dutch scientist Antonie van Leeuwenhoek changed humanity's perspective on the world with his discovery of "animalcules" in rainwater, along with their tiny appendages known as cilia today. It was marked as the first recorded observation of single-celled organisms and their locomotive structures.[30]
In the late 19th century, Karl Ernst von Baer's groundbreaking work in embryonic development laid the foundation for modern developmental biology.[31] Through meticulous observations, von Baer provided invaluable insights into tissue and organ formation during development, including the early stages of embryogenesis and the development of cilia-bearing tissues.[32] While von Baer may not have fully appreciated the significance of cilia at the time, his observations likely included their presence in embryonic tissues. Cilia - crucial for cell signaling, tissue development, and left-right asymmetry, are now recognized as ancient organelles with essential roles in development.[33] Von Baer's concept of embryonic recapitulation, despite refinement, underscores the evolutionary conservation of developmental processes, including ciliary function. Today, von Baer's legacy inspires ongoing research into embryology and developmental biology, particularly in understanding ciliary biology and its relevance to ciliopathies, where defects in ciliary structure or function lead to developmental disorder.[34]
Although non-motile or primary cilia were first described in 1898, they were largely ignored by biologists. However, microscopists continued to document their presence in the cells of most vertebrate organisms. The primary cilium was long considered—with few exceptions—to be a largely useless evolutionary vestige, a
Recent advances in mammalian genetic research have made possible the understanding of a molecular basis for a number of dysfunctional mechanisms in both motile and primary cilia structures of the cell.[36] A number of critical developmental signaling pathways essential to cellular development have been discovered. These are principally but not exclusively found in the non-motile or primary cilia. A number of common observable characteristics of mammalian genetic disorders and diseases are caused by ciliary dysgenesis and dysfunction. Once identified, these characteristics thus describe a set of hallmarks of a ciliopathy.[8]
Cilia have recently been implicated in a wide variety of human genetic diseases by "the discovery that numerous
References
- ^ PMID 18178628.
- PMID 20097287.
- PMID 24859270.
- PMID 21071979.
- ISBN 978-1-4939-7782-6.
- ISBN 978-0-19-965876-3.
- ^ PMID 16275743.
- ^ PMID 16722803.
- ^ Tan K, Liu P, Pang L, Yang W, Hou F (2018) A human ciliopathy with polycystic ovarian syndrome and multiple subcutaneous cysts: A rare case report. Medicine (Baltimore) 97(50)
- ^ ISBN 978-0-19-530016-1. Retrieved 2009-07-01.
- ^ PMID 18365235. 1432-119X.
- PMID 19439065.
- ^ a b c d "Ciliary proteome database, v3". Database introduction. Johns Hopkins University. 2008. Archived from the original on 2019-04-29. Retrieved 2009-01-07.
- PMID 17959775.
- ^ of organs The Ciliary Proteome Archived 2019-04-29 at the Wayback Machine, Ciliaproteome V3.0 - Home Page, accessed 2010-06-11.
- PMID 18528360.
- PMID 18332319.
- PMID 28698599.
- ^ PMID 35996505.
- ^ "Genetic testing for". Blueprint Genetics. Retrieved 2024-02-15.
- PMID 32055034.
- ^ "PKD1 - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2024-02-15.
- ^ PMID 19876933.
- ^ Kyttälä M (May 2006). "Identification of the Meckel Syndrome Gene (MKS1) Exposes a Novel Ciliopathy" (PDF). National Public Health Institute. Archived from the original (PDF) on 2006-07-21. Retrieved 2008-07-06.
- PMID 19876928.
- ^ Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model
- PMID 12923538.
- PMID 17520076.
- PMID 19430481.
- PMID 25960570.
- PMID 23319342.
- S2CID 9158143.
- S2CID 189898579.
- S2CID 9975220.
- ^ Gardiner MB (September 2005). "The Importance of Being Cilia". HHMI Bulletin. 18 (2). Howard Hughes Medical Institute. Archived from the original on 2010-03-11. Retrieved 2008-07-26.
- PMID 19477114.
External links
- The Ciliary Proteome Web Page at Johns Hopkins Archived 2019-04-29 at the Wayback Machine
