Circulene
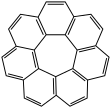
A circulene is a
synthesized include [5]circulene (corannulene), [6]circulene (coronene), [7]circulene,[2][3][4][5] and [12]circulene (kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure (compare to cones and partial cones in calixarenes). The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix
rather than a closed ring.
Quadrannulene ([4]circulene)
The simple [4]circulene compound itself has not been synthesized, but a derivative, tetrabenzo[4]circulene, also called quadrannulene, has.[6]
[8]Circulenes
The isolation of the [8]circulene derivative 2,5,6,9,10,13,14-octamethyl-3,4,7,8,11,12,15,16-octa(4-tolyl)[8]circulene has been reported.[7] Tetrabenzo[8]circulene (TB8C), a functionally stable form of the parent molecule [8]circulene has also been reported.[8][9][10]
Heterocyclic circulenes

A
heterocyclic circulene is one in which the fused rings around the periphery are not simple hydrocarbons, but instead contain at least one other element. Sulflower is a stable heterocyclic octacirculene based on thiophene
.
Gallery
-
Corannulene ([5]circulene)
-
Coronene ([6]circulene)
-
[7]circulene
-
Kekulene ([12]circulene)

![Corannulene ([5]circulene)](http://upload.wikimedia.org/wikipedia/commons/thumb/2/23/Corannulene.svg/120px-Corannulene.svg.png)
![Coronene ([6]circulene)](http://upload.wikimedia.org/wikipedia/commons/thumb/0/03/Coronene_200.svg/120px-Coronene_200.svg.png)
![[7]circulene](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d4/-7-circulene.svg/120px--7-circulene.svg.png)
![Kekulene ([12]circulene)](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/Kekulene.svg/120px-Kekulene.svg.png)