Click chemistry
| Part of a series on |
| Chemistry |
|---|
 |
In
Click reactions occur in
By developing specific and controllable
The term "click chemistry" was coined by
Background
Click chemistry is a method for attaching a
One of the earliest and most important methods in bioconjugation was to express a reporter on the same
To overcome these challenges, chemists have opted to proceed by identifying pairs of
Now limitations emerge from the chemistry of the probe to its target. In order for this technique to be useful in biological systems, click chemistry must run at or near biological conditions, produce little and (ideally) non-toxic byproducts, have (preferably) single and stable products at the same conditions, and proceed quickly to high yield in
Biotech company Shasqi is a company leveraging click chemistry in humans.[7][8]
Reactions
For a reaction to be considered a click reaction, it must satisfy certain characteristics:[9]
- modularity
- insensitivity to solvent parameters
- high chemical yields
- insensitivity towards oxygen and water
- regiospecificity and stereospecificity
- a large thermodynamic driving force (>20 kcal/mol) to favor a reaction with a single reaction product. A distinct exothermic reaction makes a reactant "spring-loaded".
The process would preferably:
- have simple reaction conditions
- use readily available starting materials and reagents
- use no solvent or use a solvent that is benign or easily removed (preferably water)
- provide simple product isolation by non-chromatographic methods (crystallisation or distillation)
- have high atom economy.
Many of the click chemistry criteria are subjective, and even if measurable and objective criteria could be agreed upon, it is unlikely that any reaction will be perfect for every situation and application. However, several reactions have been identified that fit the concept better than others:[clarification needed]
- [3+2] cycloadditions, such as the Huisgen 1,3-dipolar cycloaddition, in particular the Cu(I)-catalyzed stepwise variant,[10] are often referred to simply as Click reactions
- Thiol-ene reaction[11][12]
- [4+1] cycloadditions between isonitriles (isocyanides) and tetrazines[15]
- nucleophilic substitution especially to small strained rings like epoxy[16] and aziridines
- carbonyl-chemistry-like formation of ureas but not reactions of the aldol type due to low thermodynamic driving force.
- addition reactions to carbon-carbon double bonds like dihydroxylation or the alkynes in the thiol-yne reaction.[9]
- Sulfur (VI) Fluoride exchange
Copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)
The classic[17][18] click reaction is the copper-catalyzed reaction of an azide with an alkyne to form a 5-membered heteroatom ring: a Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). The first triazole synthesis, from diethyl acetylenedicarboxylate and phenyl azide, was reported by Arthur Michael in 1893.[19] Later, in the middle of the 20th century, this family of 1,3-dipolar cycloadditions took on Rolf Huisgen's name after his studies of their reaction kinetics and conditions.

The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery V. Fokin and

Moreover, this copper-catalyzed "click" does not require ligands on the metal, although accelerating ligands such as tris(triazolyl)methyl amine ligands with various substituents have been reported and used with success in aqueous solution.[19] Other ligands such as PPh3 and TBIA can also be used, even though PPh3 is liable to Staudinger ligation with the azide substituent. Cu2O in water at room temperature was found also to catalyze the same reaction in 15 minutes with 91% yield.[22]
The first reaction mechanism proposed included one catalytic copper atom; but isotope, kinetic, and other studies have suggested a dicopper mechanism may be more relevant.[23][24][25][26][27] Even though this reaction proceeds effectively at biological conditions, copper in this range of dosage is cytotoxic. Solutions to this problem have been presented, such as using water-soluble ligands on the copper to enhance cell penetration of the catalyst and thereby reduce the dosage needed,[28][29][30] or to use chelating ligands to further increase the effective concentration of Cu(I) and thereby decreasing the actual dosage.[31][32][33]
Although the Cu(I)-catalyzed variant was first reported by Meldal and co-workers for the synthesis of peptidotriazoles on solid support, their conditions were far from the true spirit of click chemistry and were overtaken by the publicly more recognized Sharpless. Meldal and co-workers also chose not to label this reaction type "click chemistry" which allegedly caused their discovery to be largely overlooked by the mainstream chemical society. Fokin and Sharpless independently described it as a reliable catalytic process offering "an unprecedented level of selectivity, reliability, and scope for those organic synthesis endeavors which depend on the creation of covalent links between diverse building blocks".
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.[34]
Strain-promoted azide-alkyne cycloaddition (SPAAC)
The Bertozzi group further developed one of Huisgen's copper-free click reactions to overcome the cytotoxicity of the CuAAC reaction.[35] Instead of using Cu(I) to activate the alkyne, the alkyne is instead introduced in a strained difluorooctyne (DIFO), in which the electron-withdrawing, propargylic, gem-fluorines act together with the ring strain to greatly destabilize the alkyne.[36] This destabilization increases the reaction driving force, and the desire of the cycloalkyne to relieve its ring strain.

This reaction proceeds as a concerted [3+2] cycloaddition to the triple bond in a cyclooctyne in the same mechanism as the Huisgen 1,3-dipolar cycloaddition. Substituents other than fluorines, such as benzene rings, are also allowed on the cyclooctyne.
This reaction has been used successfully to probe for azides in living systems, even though the reaction rate is somewhat slower than that of the CuAAC. Moreover, because the synthesis of cyclooctynes often gives low yield, probe development for this reaction has not been as rapid as for other reactions. But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.[37][38][39]
Strain-promoted alkyne-nitrone cycloaddition (SPANC)
Diaryl-strained-cyclooctynes including dibenzylcyclooctyne (DIBO) have also been used to react with 1,3-nitrones in strain-promoted alkyne-nitrone cycloadditions (SPANC) to yield N-alkylated isoxazolines.[40]
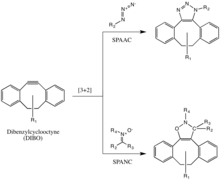
Because this reaction is metal-free and proceeds with fast kinetics (k2 as fast as 60 1/Ms, faster than both the CuAAC or the SPAAC) SPANC can be used for live cell labeling. Moreover, substitution on both the carbon and nitrogen atoms of the nitrone dipole, and acyclic and endocyclic nitrones are all tolerated. This large allowance provides a lot of flexibility for nitrone handle or probe incorporation.[41]
However, the isoxazoline product is not as stable as the triazole product of the CuAAC and the SpAAC, and can undergo rearrangements at biological conditions. Regardless, this reaction is still very useful as it has notably fast reaction kinetics.[40]
The applications of this reaction include labeling proteins containing serine as the first residue: the serine is oxidized to aldehyde with NaIO4 and then converted to nitrone with p-methoxybenzenethiol, N-methylhydroxylamine and p-ansidine, and finally incubated with cyclooctyne to give a click product. The SPANC also allows for multiplex labeling.[42][43]
Reactions of strained alkenes
Strained alkenes also utilize strain-relief as a driving force that allows for their participation in click reactions. Trans-cycloalkenes (usually cyclooctenes) and other strained alkenes such as oxanorbornadiene react in click reactions with a number of partners including azides, tetrazines and tetrazoles. These reaction partners can interact specifically with the strained alkene, staying bioorthogonal to endogenous alkenes found in lipids, fatty acids, cofactors and other natural products.[42]
Alkene and azide [3+2] cycloaddition
Oxanorbornadiene (or another activated alkene) reacts with azides, giving triazoles as a product. However, these product triazoles are not aromatic as they are in the CuAAC or SPAAC reactions, and as a result are not as stable. The activated double bond in oxanobornadiene makes a triazoline intermediate that subsequently spontaneously undergoes a retro Diels-alder reaction to release furan and give 1,2,3- or 1,4,5-triazoles. Even though this reaction is slow, it is useful because oxabornodiene is relatively simple to synthesize. The reaction is not, however, entirely chemoselective.[44]
Alkene and tetrazine inverse-demand Diels-Alder

Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro [4+2] cycloaddition (see figure).[45] Like the other reactions of the trans-cyclooctene, ring strain release is a driving force for this reaction. Thus, three-membered and four-membered cycloalkenes, due to their high ring strain, make ideal alkene substrates.[45]
Similar to other [4+2] cycloadditions, electron-donating substituents on the dienophile and electron-withdrawing substituents on the diene accelerate the inverse-demand Diels-Alder. The diene, the tetrazine, by virtue of having the additional nitrogens, is a good diene for this reaction. The dienophile, the activated alkene, can often be attached to electron-donating alkyl groups on target molecules, thus making the dienophile more suitable for the reaction.[46]
Alkene and tetrazole photoclick reaction
The tetrazole-alkene "photoclick" reaction is another dipolar addition that Huisgen first introduced in the late 1960s ChemBioChem 2007, 8, 1504. (68) Clovis, J. S.; Eckell, A.; Huisgen, R.; Sustmann, R. Chem. Ber. 1967, 100, 60.) Tetrazoles with amino or styryl groups that can be activated by UV light at 365 nm (365 does not damage cells) react quickly (so that the UV light does not have to be on for a long time, usually around 1–4 minutes) to make fluorogenic pyrazoline products. This reaction scheme is well suited for the purpose of labeling in live cells, because UV light at 365 nm damages cells minimally. Moreover, the reaction proceeds quickly, so that the UV light can be administered for short durations. Quantum yields for short wavelength UV light can be higher than 0.5. This allows tetrazoles to be used wavelength selectively in combination with another photoligation reaction, where at the short wavelength the tetrazole ligation reaction proceeds nearly exclusively and at longer wavelength another reaction (ligation via o-quinodimethanes) proceeds exclusively.[47] Finally, the non-fluorogenic reactants give rise to a fluorogenic product, equipping the reaction with a built-in spectrometry handle.
Both tetrazoles and the alkene groups have been incorporated as protein handles as unnatural amino acids, but this benefit is not unique. Instead, the photoinducibility of the reaction makes it a prime candidate for spatiotemporal specificity in living systems. Challenges include the presence of endogenous alkenes, though usually cis (as in fatty acids) they can still react with the activated tetrazole.[48]
Potential applications
Click Chemistry is a powerful tool to probe for the cellular localization of small molecules. Knowing where a small molecules goes in the cell gives powerful insights into their mechanisms of action.[49] This approach has been used in numerous studies, and discoveries include that salinomycin localizes to lysosomes to initiate ferroptosis in cancer stem cells[50] and that metformin derivatives accumulate in mitochondria to chelate copper(II), affecting metabolism and epigenetic changes downstream in inflammatory macrophages.[51]
The commercial potential of click chemistry is great. The fluorophore rhodamine has been coupled onto norbornene, and reacted with tetrazine in living systems.[52] In other cases, SPAAC between a cyclooctyne-modified fluorophore and azide-tagged proteins allowed the selection of these proteins in cell lysates.[53]
Methods for the incorporation of click reaction partners into systems in and ex vivo contribute to the scope of possible reactions. The development of unnatural amino acid incorporation by ribosomes has allowed for the incorporation of click reaction partners as unnatural side groups on these unnatural amino acids. For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid.[54] In another example, "CpK" has a side group including a cyclopropane alpha to an amide bond that serves as a reaction partner to tetrazine in an inverse diels-alder reaction.[55]

The synthesis of luciferin exemplifies another strategy of isolating reaction partners, which is to take advantage of rarely-occurring, natural groups such as the 1,2-aminothiol, which appears only when a cysteine is the final N' amino acid in a protein. Their natural selectivity and relative bioorthogonality is thus valuable in developing probes specific for these tags. The above reaction occurs between a 1,2-aminothiol and a 2-cyanobenzothiazole to make luciferin, which is fluorescent. This luciferin fluorescence can be then quantified by spectrometry following a wash, and used to determine the relative presence of the molecule bearing the 1,2-aminothiol. If the quantification of non-1,2-aminothiol-bearing protein is desired, the protein of interest can be cleaved to yield a fragment with a N' Cys that is vulnerable to the 2-CBT.[56]
Additional applications include:
- two-dimensional gel electrophoresis separation[57]
- preparative organic synthesis of 1,4-substituted triazoles
- modification of peptide function with triazoles
- modification of natural products and pharmaceuticals
- natural product discovery [58]
- drug discovery
- macrocyclizations using Cu(I) catalyzed triazole couplings
- modification of DNA and nucleotides by triazole ligation
- supramolecular chemistry: calixarenes, rotaxanes, and catenanes
- dendrimer design
- carbohydrate clusters and carbohydrate conjugation by Cu(1) catalyzed triazole ligation reactions
- biopolymers[59]
- surfaces[60]
- material science
- nanotechnology,[61]
- bioconjugation, for example, azidocoumarin, and
- biomaterials[62]
In combination with
Technology license
The Scripps Research Institute has a portfolio of click-chemistry patents.[63] Licensees include Invitrogen,[64] Allozyne,[65] Aileron,[66] Integrated Diagnostics,[67] and the biotech company baseclick,[68] a BASF spin-off created to sell products made using click chemistry.[69] Moreover, baseclick holds a worldwide exclusive license for the research and diagnostic market for the nucleic acid field. Fluorescent azides and alkynes are also produced by companies such as Cyandye.[70]
References
- ^
Carroll, G.T.; London, G.; Fernandez-Landaluce, T.; Rudolf, P.; Feringa, B.L. (2011). "Adhesion of Photon-Driven Molecular Motors to Surfaces via 1,3-Dipolar Cycloadditions: Effect of Interfacial Interactions on Molecular Motion" (PDF). ACS Nano. 5 (1): 622–630. S2CID 39105918.
- ^ Nobel Prize lecture: Barry Sharpless, Nobel Prize in Chemistry 2022, retrieved 2024-01-04
- PMID 11433435.
- doi:10.1071/CH06457.
- ^ "The Nobel Prize in Chemistry 2022". NobelPrize.org. Retrieved 2022-10-05.
- S2CID 248494718.
- ^ "The bioorthogonal revolution". Chemistry World. Retrieved 2022-11-11.
- ^ "'Honeymoon-Phase' Chemical Partners Deliver a Toxic Drug to Tumors". Discover Magazine. Retrieved 2022-11-11.
- ^ S2CID 104470679.
- ^
Spiteri, Christian; Moses, John E. (2010). "Copper-Catalyzed Azide–Alkyne Cycloaddition: Regioselective Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles". Angewandte Chemie International Edition. 49 (1): 31–33. PMID 19921729.
- ^
Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1540–1573. PMID 20166107.
- ^ Lowe, A. B. Polymer Chemistry 2010, 1 (1), 17–36. DOI: 10.1039/B9PY00216B
- PMID 18798613.
- PMID 19053305.
- ^
Stöckmann, Henning; Neves, Andre; Stairs, Shaun; Brindle, Kevin; Leeper, Finian (2011). "Exploring isonitrile-based click chemistry for ligation with biomolecules". PMID 21915395.
- ^
Kashemirov, Boris A.; Bala, Joy L. F.; Chen, Xiaolan; Ebetino, F. H.; Xia, Zhidao; Russell, R. Graham G.; Coxon, Fraser P.; Roelofs, Anke J.; Rogers Michael J.; McKenna, Charles E. (2008). "Fluorescently labeled risedronate and related analogues: "magic linker" synthesis". PMID 19032080.
- ^ Gregory C., Patton (November 8, 2004). "Development and Applications of Click Chemistry" (PDF). Department of Chemistry. College of Liberal Arts & Sciences, University of Illinois at Urbana-Champaign. Archived from the original (PDF) on 2010-07-09.
{{cite journal}}: Cite journal requires|journal=(help) - PMID 14678739.
- ^ a b c L. Liang and D. Astruc: "The copper(I)-catalysed alkyne-azide cycloaddition (CuAAC) "click" reaction and its applications. An overview", 2011; 255, 23–24, 2933–2045, p. 2934
- PMID 12203546.
- ^
Tornoe, C. W.; Christensen, C.; Meldal, M. (2002). "Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides". S2CID 11957672.
- ^ K. Wang, X. Bi, S. Xing, P. Liao, Z. Fang, X. Meng, Q. Zhang, Q. Liu, Y. Ji Green Chem., 13 (2011), p. 562
- ^ B. T. Worrell, J. A. Malik, V. V. Fokin 2013, 340, 457–459 ; J.E. Hein, V.V. Fokin, Chem. Soc. Rev. 39 (2010) 1302.
- PMID 15693051.
- PMID 25614295.
- ISSN 0276-7333.
- PMID 28394586.
- ^ Brotherton, W. S.; Michaels, H. A.; Simmons, J. T.; Clark, R.J.; Dalal, N. S.; Zhu, L. Org. Lett. 2009, 11, 4954.
- ^ Kuang, G.-C.; Michaels, H. A.; Simmons, J. T.; Clark, R. J.; Zhu, L" J. Org. Chem. 2010; 75, 6540.
- ^ Uttamapinant, C.; Tangpeerachaikul, A.; Grecian, S.; Clarke, S.; Singh, U.; Slade, P.; Gee, K. R.; Ting, A. Y" Angew. Chem. Int. Ed. 2012; 51, 5852
- ^ Alder, K.; Stein, G.; Finzenhagen, H. Justus Liebigs Ann.Chem 1931, 485, 211.
- ^ Alder, K.; Stein, G. Justus Liebigs Ann. Chem. 1933, 501, 1.
- ^ Wittig, G.; Krebs, A. Chem. Ber. 1961, 94, 3260.
- PMID 16287266.
- PMID 17175580.
- PMID 17175580.
- PMID 18680289.
- PMID 18275058.
- PMID 22553995.
- ^ PMID 25022431.
- ^ (64) (a) Ning, X.; Temming, R. P.; Dommerholt, J.; Guo, J.; Ania, D.B.; Debets, M. F.; Wolfert, M. A.; Boons, G.-J.; van Delft, F. L" Angew. Chem. Int. Ed. 2010; 49, 3065. (b) McKay, C. S.; Moran, J.; Pezacki, J. P. Chem. Commun. (Cambridge, U. K.) 2010, 46, 931. (c) Debets, M. F.; van Berkel, S. S.; Dommerholt, J.; Dirks, A. T. J.; Rutjes, F. P. J. T.; van Delft, F. L. Acc. Chem. Res. 2011, 44, 805. (d) McKay, C. S.; Chigrinova, M.; Blake, J. A.; Pezacki, J. P. Org. Biomol. Chem. 2012, 10, 3066.
- ^ PMID 24432752.
- .
- ^ (67) (a) van Berkel, S. S.; Dirks, A. T. J.; Meeuwissen, S. A.; Pingen, D. L. L.; Boerman, O. C.; Laverman, P.; van Delft, F. L.; Cornelissen, J. J. L. M.; Rutjes, F. P. J. T. ChemBioChem 2008, 9, 1805. (b) van Berkel, S. S.; Dirks, A. T. J.; Debets, M. F.; van Delft, F. L.; Cornelissen, J. J. L. M.; Nolte, R. J. M.; Rutjes, F. P. J. T. ChemBioChem 2007, 8, 150
- ^ PMID 24044412.
- PMID 24981416.
- PMID 30916368.
- PMID 25022432.
- S2CID 91366817.
- PMID 28937680.
- PMID 37100912.
- PMID 19053305.
- PMID 10805777.
- S2CID 2833184.
- PMID 22997015.
- ^ (a) Liang, G.; Ren, H.; Rao, J. Nat. Chem. 2010, 2, 54. (b) Ren, H.; Xiao, F.; Zhan, K.; Kim, Y.-P.; Xie, H.; Xia, Z.; Rao, J. Angew.Chem., Int. Ed. 2009, 48, 9658.
- S2CID 9002232. Archived from the original(PDF) on 2015-06-30. Retrieved 2015-02-11.
- PMID 24937678.
- PMID 25032224.
- S2CID 5759186.
- PMID 17619685.
- PMID 18406491.
- ^ "Click Chemistry". Archived from the original on 2012-05-15. Retrieved 2012-06-05.
- ^ "Invitrogen Exclusively Licenses Novel Click Chemistry-Based Cell Proliferation Assays from Harvard University (NASDAQ:LIFE)". Archived from the original on 2012-12-17. Retrieved 2012-06-05.
- ^ "Xconomy: Allozyne Licenses Scripps Chemistry". 2010-07-15.
- ^ "Xconomy: Aileron and Scripps Ink Deal". 2010-11-30.
- ^ "Integrated Diagnostics Licenses "Click Chemistry" from the Scripps Research Institute, Strengthening Partner Network". Archived from the original on 2012-04-30. Retrieved 2012-06-05.
- ^ "baseclick GmbH :: We enable nucleic acid labeling bioconjugation". baseclick GmbH. Retrieved 2022-03-21.
- ^ http://www.basf.com/group/pressrelease/P-10-427 [permanent dead link]
- ^ "CYANDYE". 2018-10-03. Archived from the original on 3 October 2018. Retrieved 2022-03-21.
External links
- Click Chemistry: Short Review and Recent Literature
- National Science Foundation: Feature "Going Live with Click Chemistry"
- Chemical and Engineering News: Feature "In-Situ Click Chemistry"
- Chemical and Engineering News: Feature "Copper-free Click Chemistry"
- Metal-free click chemistry review
- Click Chemistry – a Chem Soc Rev themed issue highlighting the latest applications of click chemistry, guest edited by M. G. Finn and Valery Fokin. Published by the Royal Society of Chemistry
