Cloperastine
 | |
| Clinical data | |
|---|---|
| Other names | HT-11 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Cloperastine (
cough suppressant in Japan, Hong Kong, and in some European countries.[1][2][3] It was first introduced in 1972 in Japan, and then in Italy in 1981.[4]
Side effects
Adverse effects may include sedation, drowsiness, heartburn, and thickening of bronchial secretions.[5]
Pharmacology
The precise
H1 receptor),[3][6] and anticholinergic.[3][11] It is thought that the latter two properties contribute to side effects, such as sedation and somnolence, while the former two may be involved in or responsible for the antitussive efficacy of cloperastine.[6][7]
Synthesis
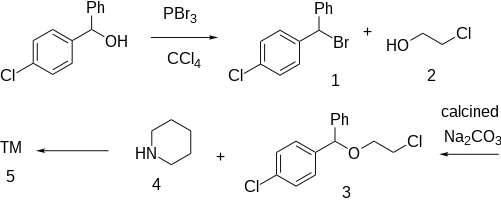
The halogenation of 4-Chlorobenzhydrol [119-56-2] (1) with
tetrachloromethane gives 1-(Bromophenylmethyl)-4-chlorobenzene [948-54-9] (2). Treatment with ethylenechlorohydrin (2-Chloroethanol) [107-07-3] (3) gives 1-(4-Chlorobenzhydryl)oxy-2-chloroethane [5321-46-0] (4). Reaction with piperidine
(5) completes the synthesis of Cloperastine (6).
See also
- Cough syrup
- Noscapine
- Codeine; Pholcodine
- Dextromethorphan; Dimemorfan
- Racemorphan; Dextrorphan; Levorphanol
- Butamirate
- Pentoxyverine
- Tipepidine
- Levocloperastine
References
- ISBN 978-1-4757-2085-3.
- ISBN 978-3-88763-075-1.
- ^ PMID 21445282.
- ISBN 978-0-8155-1856-3.
- ISBN 0-8103-7177-4.
- ^ PMID 22711801.
- ^ ISBN 9783540798422.
- S2CID 30118634.
- PMID 21467791.
- PMID 20460867.
- ISBN 978-0-471-88356-2.
- PMID 13159698.
- ^ Anon., GB 1179945 (1970 to Yoshitomi Pharmaceutical).
- ^ Anon., GB 670622 (1952 to Parke Davis & Co).
- ^ Laura Puricelli, EP 0894794 (1999 to AESCULAPIUS FARMACEUTICI S.r.l.).
- ^ 陶文潘, 潘文驰, 潘兴长, 罗泳萍, 樊希祥, CN 104327014A (2015 to 重庆市恒安化工有限公司).
