Cyclic compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds with rings containing both carbon and non-carbon). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many billions.
- Cyclic compound examples: All-carbon (carbocyclic) and more complex natural cyclic compounds
-
that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-aromatic, carbocyclic rings.
-
Cycloalkanes, the simplest carbocycles, including cyclopropane, cyclobutane, cyclopentane, and cyclohexane. Note, elsewhere an organic chemistry shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly).
-
Paclitaxel, another complex, plant-derived terpenoid, also a natural product, displaying a complex multi-ring structure including 4-, 6-, and 8-membered rings (carbocyclic and heterocyclic, aromatic and non-aromatic).
Adding to their complexity and number, closing of atoms into rings may lock particular atoms with distinct substitution (by functional groups) such that stereochemistry and chirality of the compound results, including some manifestations that are unique to rings (e.g., configurational isomers). As well, depending on ring size, the three-dimensional shapes of particular cyclic structures – typically rings of five atoms and larger – can vary and interconvert such that conformational isomerism is displayed. Indeed, the development of this important chemical concept arose historically in reference to cyclic compounds. Finally, cyclic compounds, because of the unique shapes, reactivities, properties, and bioactivities that they engender, are the majority of all molecules involved in the biochemistry, structure, and function of living organisms, and in man-made molecules such as drugs, pesticides, etc.
Structure and classification
A cyclic compound or ring compound is a compound in which at least some its atoms are connected to form a ring.[1] Rings vary in size from three to many tens or even hundreds of atoms. Examples of ring compounds readily include cases where:
- all the atoms are carbon (i.e., are carbocycles),
- none of the atoms are carbon (inorganic cyclic compounds),[2] or where
- both carbon and non-carbon atoms are present (heterocycliccompounds with rings containing both carbon and non-carbon).
Common atoms can (as a result of their
Moreover, the closing of atoms into rings may lock particular
Carbocycles
The vast majority of cyclic compounds are organic, and of these, a significant and conceptually important portion are composed of rings made only of carbon atoms (i.e., they are carbocycles).[citation needed]
Inorganic cyclic compounds
Inorganic atoms form cyclic compounds as well. Examples include
Heterocyclic compounds
A heterocyclic compound is a cyclic compound that has atoms of at least two different
Macrocycles

The term
Nomenclature
The atoms that are part of the ring structure are called annular atoms.[10]
Isomerism
Stereochemistry
The closing of atoms into rings may lock particular atoms with distinct
Conformational isomerism
Depending on ring size, the three-dimensional shapes of particular cyclic structures—typically rings of 5-atoms and larger—can vary and interconvert such that
The chair conformation is the favored configuration, because in this conformation, the
Aromaticity
copying within Wikipedia " guideline for an overview of the issues involved. |
Cyclic compounds may or may not exhibit aromaticity; benzene is an example of an aromatic cyclic compound, while cyclohexane is non-aromatic. In organic chemistry, the term aromaticity is used to describe a cyclic (ring-shaped), planar (flat) molecule that exhibits unusual stability as compared to other geometric or connective arrangements of the same set of atoms. As a result of their stability, it is very difficult to cause aromatic molecules to break apart and to react with other substances. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have especial stability (low reactivity).
Since one of the most commonly encountered aromatic systems of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA. A functional group or other substituent that is aromatic is called an aryl group.
The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (aromas), unlike pure saturated hydrocarbons. Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds (how they smell), although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.
In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often made of alternating single and double bonds in a ring. This configuration allows for the electrons in the molecule's pi system to be delocalized around the ring, increasing the molecule's stability. The molecule cannot be represented by one structure, but rather a resonance hybrid of different structures, such as with the two resonance structures of benzene. These molecules cannot be found in either one of these representations, with the longer single bonds in one location and the shorter double bond in another (See Theory below). Rather, the molecule exhibits bond lengths in between those of single and double bonds. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.[citation needed]
Principal uses
Because of the unique shapes, reactivities, properties, and
Synthetic reactions
Important general reactions for forming rings

There are a variety of specialized reactions whose use is solely the formation of rings, and these will be discussed below. In addition to those, there are a wide variety of general organic reactions that historically have been crucial in the development, first, of understanding the concepts of ring chemistry, and second, of reliable procedures for preparing ring structures in high
- Acyloin condensation;
- Anodic oxidations; and
- the Dieckmann condensation as applied to ring formation.
Ring-closing reactions
In organic chemistry, a variety of synthetic procures are particularly useful in closing carbocyclic and other rings; these are termed ring-closing reactions. Examples include:
- alkyne trimerisation;
- the Bergman cyclization of an enediyne;
- the Diels–Alder, between a conjugated diene and a substituted alkene, and other cycloaddition reactions;
- the Nazarov cyclization reaction, originally being the cyclization of a divinyl ketone;
- various radical cyclizations;
- ring-closing metathesis reactions, which also can be used to accomplish a specific type of polymerization;
- the carbonylgroup with loss of CO2 and H2O;
- the amino alcohol to an aziridine
- other reactions, such as an amino group reacting with a hydroxy group, as in the biosynthesis of solanine
Ring-opening reactions
A variety of further synthetic procedures are particularly useful in opening carbocyclic and other rings, generally which contain a double bound or other functional group "handle" to facilitate chemistry; these are termed ring-opening reactions. Examples include:
- ring opening metathesis, which can also be used to accomplish a specific type of polymerization.
Ring expansion and ring contraction reactions
Ring expansion and contraction reactions are common in
Examples
Simple, mono-cyclic examples
The following are examples of simple and aromatic carbocycles, inorganic cyclic compounds, and heterocycles:
- Simple mono-cyclic compounds: Carbocyclic, inorganic, and heterocyclic (aromatic and non-aromatic) examples.
-
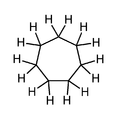
Cycloheptane, a simple 7-membered carbocyclic compound, methylene hydrogens shown (non-aromatic).
-
delocalizedthrough drawing of circle (aromatic).
-
Cyclo-octasulfur, an 8-membered inorganic cyclic compound (non-aromatic).
-
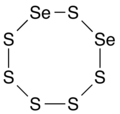
Diselenium hexasulfide, an 8-membered inorganic heterocyclic compound (non-aromatic).
-
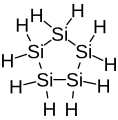
Cyclopentasilane, a 5-membered inorganic cyclic compound (non-aromatic).
-
Hexamethylcyclotrisiloxane, a 6-membered organic heterocyclic compound (non-aromatic).
-
Hexachlorophosphazene, a 6-membered inorganic heterocyclic compound (aromatic).
-
Borazine, a 6-membered inorganic heterocyclic compound (may be aromatic).
-
Pentazole, a 5-membered inorganic cyclic compound (aromatic).
-
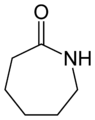
Caprolactam, a 7-membered heterocyclic organic compound (non-aromatic).
-
delocalizedπ-electrons shown as discrete bonds (aromatic).
Complex and polycyclic examples
The following are examples of cyclic compounds exhibiting more complex ring systems and stereochemical features:
- Complex cyclic compounds: Macrocyclic and polycyclic examples
-
delocalizationof π-electrons (aromatic).
-
Decalin (decahydronaphthalene), the fully saturated derivative of naphthalene, showing the two stereochemistries possible for "fusing" the two rings together, and how this impacts the shapes available to this bicyclic compound (non-aromatic).
-
phenyl-rings on its "tail", and attached to C-2 (abbrev. Ph, C6H5; aromatics).
-
A representative three-dimensional shape adopted by paclitaxel, as a result of its unique cyclic structure.[11]
-
Cholesterol, another terpene natural product, in particular, a steroid, a class of tetracyclic molecules (non-aromatic).
-
delocalizedπ-electrons shown as discrete bonds (aromatic).
-
Pagodane, a complex, highly symmetric, man-made polycyclic compound (non-aromatic).
-
red tides. The R group at right refers to one of several possible four-carbon side chains (see main Brevetoxinarticle; non-aromatic).
See also
References
- OCLC 642506595[page needed]
- S2CID 93804259.
- PMID 25687211.
- ^ a b c d e f g William Reusch (2010). "Stereoisomers Part I" in Virtual Textbook of Organic Chemistry. Michigan State University. Archived from the original on 10 March 2015. Retrieved 7 April 2015.
- ^ IUPAC Gold Book heterocyclic compounds
- ^ .
- ^ a b J. D. Dunitz (1968). J. D. Dunitz and J. A. Ibers (ed.). Perspectives in Structural Chemistry. Vol. 2. New York: Wiley. pp. 1–70.
- ^ Eliel, E.L., Wilen, S.H. and Mander, L.S. (1994) Stereochemistry of Organic Compounds, John Wiley and Sons, Inc., New York.[page needed]
- .
- ISBN 9780122004001. Archivedfrom the original on 2021-04-13. Retrieved 2020-09-14.
- from the original on 2021-01-22. Retrieved 2020-09-14.
Further reading
- Jürgen-Hinrich Fuhrhop & Gustav Penzlin, 1986, "Organic synthesis: concepts, methods, starting materials," Weinheim, BW, DEU:VCH, ISBN 0895732467, see [1], accessed 19 June 2015.
- Michael B. Smith & Jerry March, 2007, "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure," 6th Ed., New York, NY, USA:Wiley & Sons, ISBN 0470084944, see [2], accessed 19 June 2015.
- Francis A. Carey & Richard J. Sundberg, 2006, "Title Advanced Organic Chemistry: Part A: Structure and Mechanisms," 4th Edn., New York, NY, USA:Springer Science & Business Media, ISBN 0306468565, see [3], accessed 19 June 2015.
- Michael B. Smith, 2011, "Organic Chemistry: An Acid—Base Approach," Boca Raton, FL, USA:CRC Press, ISBN 1420079212, see [4], accessed 19 June 2015. [May not be most necessary material for this article, but significant content here is available online.]
- Jonathan Clayden, Nick Greeves & Stuart Warren, 2012, "Organic Chemistry," Oxford, Oxon, GBR:Oxford University Press, ISBN 0199270295, see [5], accessed 19 June 2015.
- László Kürti & Barbara Czakó, 2005, "Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms, Amsterdam, NH, NLD:Elsevier Academic Press, 2005ISBN 0124297854, see [6], accessed 19 June 2015.
External links
- Polycyclic+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Macrocyclic+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)

















![A representative three-dimensional shape adopted by paclitaxel, as a result of its unique cyclic structure.[11]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5a/Paclitaxel_JMolBiol_2001_1045.jpg/108px-Paclitaxel_JMolBiol_2001_1045.jpg)

![Benzo[a]pyrene, a pentacyclic compound both natural and man-made, and delocalized π-electrons shown as discrete bonds (aromatic).](http://upload.wikimedia.org/wikipedia/commons/thumb/f/fa/Benzo-a-pyrene.svg/192px-Benzo-a-pyrene.svg.png)

