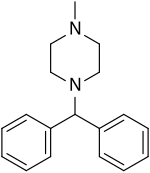
Cyclizine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Marezine, Valoid, Nausicalm, others |
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Routes of administration | By mouth, IM, IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | N-demethylated to inactive norcyclizine[1] |
| Elimination half-life | 20 hours |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Cyclizine, sold under a number of brand names, is a medication used to treat and prevent
Common side effects include sleepiness, dry mouth,
Cyclizine was discovered in 1947.
Medical uses
Primary uses include
The drug Diconal is a combination of cyclizine and the opioid
Contraindications
Its antimuscarinic action warrants caution in patients with prostatic hypertrophy, urinary retention, or angle-closure glaucoma. Liver disease exacerbates its sedative effects.[10]
Adverse effects
Common (over 10%) —
Uncommon (1% to 10%) — Headache, psychomotor impairment, dermatitis, and
Rare (less than 1%) — Hypersensitivity reactions (bronchospasm, angioedema, anaphylaxis, rashes and photosensitivity reactions), extrapyramidal effects, dizziness, confusion, depression, sleep disturbances, tremor, liver dysfunction, and hallucinations.
Pharmacology
Cyclizine is a
Synthesis
Cyclizine may be prepared by the
History
Cyclizine was developed in the American division of pharmacy company
The substance received more credit when NASA chose it as a space antiemetic for the first crewed Moon flight. Cyclizine was introduced to many countries as a common antiemetic. It is an over-the-counter drug in many countries because it has been well tolerated, although it has not been studied much.[13][15]
Society and culture
Some people using methadone recreationally combine cyclizine with their
It has been used illegally in greyhound racing to sabotage a dog's performance.[18]
Names
As cyclizine hydrochloride tablets and cyclizine lactate solution for intramuscular or intravenous injection (brand names: Valoid[10] in UK and South Africa and Marezine, Marzine and Emoquil in US). Cyclizine was marketed as Bonine for Kids in the US, but was discontinued in 2012, and replaced with meclizine.[19]
Cyclizine derivatives
| Structural comparison of cyclizine and related H1 antagonists[20] | ||
|---|---|---|

| ||
| Compound | R1 | R2 |
| Cyclizine | H | CH3
|
| Chlorcyclizine | Cl | CH3 |
| Meclizine | Cl | 
|
| Buclizine | Cl | 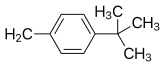
|
| Oxatomide | H | 
|
| Hydroxyzine | Cl | |
| Cetirizine | Cl | 
|
See also
References
- ^ "DrugBank: Cyclizine. Pharmacology: metabolism". DrugBank Database. Archived from the original on 30 January 2016. Retrieved 5 January 2016.
- ^ a b c "Cyclizine 50mg Tablets - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. 27 March 2015. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
- ^ ISBN 9781455749898. Archivedfrom the original on 20 December 2016.
- hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b "Cyclizine Side Effects in Detail - Drugs.com". www.drugs.com. Archived from the original on 21 December 2016. Retrieved 13 December 2016.
- ^ a b c "Cyclizine: Indications, Side Effects, Warnings - Drugs.com". www.drugs.com. Archived from the original on 21 December 2016. Retrieved 13 December 2016.
- ^ "Cyclizine Use During Pregnancy | Drugs.com". www.drugs.com. Archived from the original on 21 December 2016. Retrieved 13 December 2016.
- ISBN 9781899163922. Archivedfrom the original on 20 December 2016.
- hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ a b c d "Valoid Tablets by Amdipharm". Electronic Medicines Compendium. Datapharm. Archived from the original on 4 March 2016. Retrieved 1 October 2011.
- ^ "Diconal Tablets by Amdipharm". Electronic Medicines Compendium. Datapharm. Archived from the original on 1 April 2008. Retrieved 5 August 2008.
- ^ "Conversion Factors for Controlled Substances". DEA Diversion Control Division. Retrieved 26 March 2020.
- ^ a b
Sneader W (2005). Drug discovery: a history. John Wiley & Sons. p. 404. ISBN 0-471-89979-8. Archivedfrom the original on 10 September 2017.
- ^
Sittig M (1988). Pharmaceutical manufacturing encyclopedia. William Andrew. p. 406. ISBN 0-8155-1144-2. Archivedfrom the original on 10 September 2017.
- ^ Rajoo SG. "Introduction". In Lobo M (ed.). Anti-emetics - Metoclopramide, Domperidone, Ondansetron, Cyclizine. Archived from the original on 2 April 2015. Retrieved 9 March 2015.
{{cite book}}:|work=ignored (help) - PMID 2775912.
- PMID 8765114.
- ^ Conor Ryan for The Independent. June 20, 2013 IGB left with €250k bill after dog doping case Archived 2016-03-04 at the Wayback Machine
- ^ "Bonine for Kids". Insight Pharmaceuticals. Archived from the original on 17 September 2010.
- ISBN 978-1-60913-345-0.
External links
- "Cyclizine". Drug Information Portal. U.S. National Library of Medicine.
