Deep vein thrombosis
| Deep vein thrombosis | |
|---|---|
| Other names | Deep venous thrombosis |
unfractionated heparin, warfarin | |
| Frequency | From 0.8–2.7 per 1000 people per year, but populations in China and Korea are below this range[8] |
Deep vein thrombosis (DVT) is a type of
The most common life-threatening concern with DVT is the potential for a clot to
The mechanism behind DVT formation typically involves some combination of
People suspected of having DVT can be assessed using a
Using
Signs and symptoms
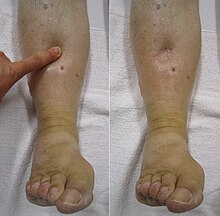
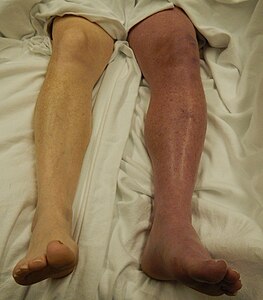
Symptoms classically affect a leg and typically develop over hours or days,[20] though they can develop suddenly or over a matter of weeks.[21] The legs are primarily affected, with 4–10% of DVT occurring in the arms.[11] Despite the signs and symptoms being highly variable,[5] the typical symptoms are pain, swelling, and redness. However, these symptoms might not manifest in the lower limbs of those unable to walk.[22] In those who are able to walk, DVT can reduce one's ability to do so.[23] The pain can be described as throbbing and can worsen with weight-bearing, prompting one to bear more weight with the unaffected leg.[21][24] Additional signs and symptoms include tenderness, pitting edema (see image), dilation of surface veins, warmth, discoloration, a "pulling sensation", and even cyanosis (a blue or purplish discoloration) with fever.[5][20][21] DVT can also exist without causing any symptoms.[22] Signs and symptoms help in determining the likelihood of DVT, but they are not used alone for diagnosis.[19]
At times, DVT can cause symptoms in both arms or both legs, as with bilateral DVT.
Potential complications
A
A rare and massive DVT that causes significant obstruction and discoloration (including cyanosis) is phlegmasia cerulea dolens.[31][32] It is life-threatening, limb-threatening, and carries a risk of venous gangrene.[33] Phlegmasia cerulea dolens can occur in the arm but more commonly affects the leg.[34][35] If found in the setting of acute compartment syndrome, an urgent fasciotomy is warranted to protect the limb.[36] Superior vena cava syndrome is a rare complication of arm DVT.[11]
DVT is thought to be able to cause a
-
A CT image with red arrows indicating PE (grey) in thepulmonary arteries(white)
-
A case of phlegmasia cerulea dolens in the left leg
-
A depiction of a patent foramen ovale
Differential diagnosis
In most suspected cases, DVT is ruled out after evaluation.
Classification
DVT and PE are the two manifestations of the
DVT is classified as
DVT in a leg above the knee is termed proximal DVT (
DVT can be classified into provoked and unprovoked categories.[52] For example, DVT that occurs in association with cancer or surgery can be classified as provoked.[52] However, the European Society of Cardiology in 2019 urged for this dichotomy to be abandoned to encourage more personalized risk assessments for recurrent VTE.[53] The distinction between these categories is not always clear.[54]
Causes
Traditionally, the three factors of
Acquired risk factors include the strong risk factor of older age,
Infections, including
Cancer can grow in and around veins, causing venous stasis, and can also stimulate increased levels of tissue factor.

Dozens of genetic risk factors have been identified,
Blood alterations including dysfibrinogenemia,[65] low free protein S,[58] activated protein C resistance,[58] homocystinuria,[92] hyperhomocysteinemia,[62] high fibrinogen levels,[62] high factor IX levels,[62] and high factor XI levels[62] are associated with increased risk. Other associated conditions include heparin-induced thrombocytopenia, catastrophic antiphospholipid syndrome,[93] paroxysmal nocturnal hemoglobinuria,[94] nephrotic syndrome,[58] chronic kidney disease,[95] polycythemia vera, essential thrombocythemia,[96] intravenous drug use,[97] and smoking.[d]
Some risk factors influence the location of DVT within the body. In isolated distal DVT, the profile of risk factors appears distinct from proximal DVT. Transient factors, such as surgery and immobilization, appear to dominate, whereas thrombophilias[e] and age do not seem to increase risk.[101] Common risk factors for having an upper extremity DVT include having an existing foreign body (such as a central venous catheter, a pacemaker, or a triple-lumen PICC line), cancer, and recent surgery.[11]
Pathophysiology

Blood has a natural tendency to clot when blood vessels are damaged (
DVT often develops in the calf veins and "grows" in the direction of venous flow, towards the heart.[42][103] DVT most frequently affects veins in the leg or pelvis[9] including the popliteal vein (behind the knee), femoral vein (of the thigh), and iliac veins of the pelvis. Extensive lower-extremity DVT can even reach into the inferior vena cava (in the abdomen).[104] Upper extremity DVT most commonly affects the subclavian, axillary, and jugular veins.[11]
The process of fibrinolysis, where DVT clots can be dissolved back into the blood, acts to temper the process of thrombus growth.
In arterial thrombosis, blood vessel wall damage is required, as it initiates
Tissue factor, via the tissue factor–

Often, DVT begins in the valves of veins.
Diagnosis
A
While the Wells score is the predominant and most studied clinical prediction rule for DVT,
| Criteria | Wells score for DVT[g] | Dutch Primary Care Rule |
|---|---|---|
| Active cancer (treatment within last 6 months or palliative) | +1 point | +1 point |
| Calf swelling ≥ 3 cm compared to asymptomatic calf (measured 10 cm below tibial tuberosity )
|
+1 point | +2 points |
| Swollen unilateral superficial veins (non-varicose, in symptomatic leg) | +1 point | +1 point |
| Unilateral pitting edema (in symptomatic leg) | +1 point | — |
| Previous documented DVT | +1 point | — |
| Swelling of entire leg | +1 point | — |
| Localized tenderness along the deep venous system | +1 point | — |
| Paralysis, paresis, or recent cast immobilization of lower extremities | +1 point | — |
| Recently bedridden ≥ 3 days, or major surgery requiring regional or general anesthetic in the past 12 weeks | +1 point | +1 point |
| Alternative diagnosis at least as likely | −2 points | — |
| Positive D-dimer (≥ 0.5 mcg/mL or 1.7 nmol/L) | — | +6 points |
| Absence of leg trauma | — | +1 point |
| Male sex | — | +1 point |
| Use of oral contraceptives | — | +1 point[5][116] |
Compression
-
An ultrasound with a blood clot visible in the left common femoral vein. (The common femoral vein is distal to the external iliac vein.)
-
Doppler ultrasonography showing absence of flow and hyperechogenic content in a clotted femoral vein (labeled subsartorial[h]) distal to the branching point of the deep femoral vein. When compared to this clot, clots that instead obstruct the common femoral vein (proximal to this branching point) cause more severe effects due to impacting a significantly larger portion of the leg.[122]
-
An abdominal CT scan demonstrating an iliofemoral DVT, with the clot in the right common iliac vein of the pelvis
Management
Treatment for DVT is warranted when the clots are either proximal, distal and symptomatic, or upper extremity and symptomatic.[2] Providing anticoagulation, or blood-thinning medicine, is the typical treatment after patients are checked to make sure they are not subject to bleeding.[2][i] However, treatment varies depending upon the location of DVT. For example, in cases of isolated distal DVT, ultrasound surveillance (a second ultrasound after 2 weeks to check for proximal clots), might be used instead of anticoagulation.[5][124] Although, those with isolated distal DVT at a high-risk of VTE recurrence are typically anticoagulated as if they had proximal DVT. Those at a low-risk for recurrence might receive a four to six week course of anticoagulation, lower doses, or no anticoagulation at all.[5] In contrast, those with proximal DVT should receive at least 3 months of anticoagulation.[5]
Some anticoagulants can be taken by mouth, and these oral medicines include
The duration of anticoagulation therapy (whether it will last 4 to 6 weeks,
Treatment for acute leg DVT is suggested to continue at home for uncomplicated DVT instead of hospitalization. Factors that favor hospitalization include severe symptoms or additional medical issues.[12] Early walking is suggested over bedrest.[134] Graduated compression stockings—which apply higher pressure at the ankles and a lower pressure around the knees[126] can be trialed for symptomatic management of acute DVT symptoms, but they are not recommended for reducing the risk of post-thrombotic syndrome,[125] as the potential benefit of using them for this goal "may be uncertain".[5] Nor are compression stockings likely to reduce VTE recurrence.[135] They are, however, recommended in those with isolated distal DVT.[5]
If someone decides to stop anticoagulation after an unprovoked VTE instead of being on lifelong anticoagulation, aspirin can be used to reduce the risk of recurrence,[136] but it is only about 33% as effective as anticoagulation in preventing recurrent VTE.[52] Statins have also been investigated for their potential to reduce recurrent VTE rates, with some studies suggesting effectiveness.[137]
Investigations for cancer
An unprovoked VTE might signal the presence of an unknown cancer, as it is an underlying condition in up to 10% of unprovoked cases.
Interventions
A mechanical thrombectomy device can remove DVT clots, particularly in acute iliofemoral DVT (DVT of the major veins in the pelvis), but there is limited data on its efficacy. It is usually combined with thrombolysis, and sometimes temporary IVC filters are placed to protect against PE during the procedure.
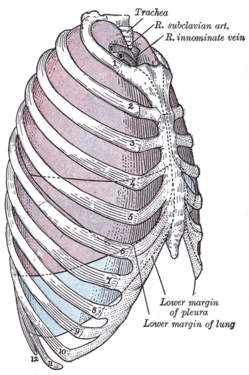
In DVT in the arm, the first (topmost) rib can be surgically removed as part of the typical treatment when the DVT is due to
-
The first rib, which is removed in a first rib resection surgery, is labeled 1 in this image
-
A venogram before catheter-directed thrombolysis forPaget–Schroetter syndrome, a rare and severe arm DVT shown here in a judo practitioner, with highly restricted blood flow shown in the vein
-
After treatment with catheter-directed thrombolysis, blood flow in thesequelae from thoracic outlet compression.[147]

The placement of an
Field of medicine
Patients with a history of DVT might be managed by
Prevention
For the
Hospital (non-surgical) patients
Acutely ill hospitalized patients are suggested to receive a parenteral anticoagulant, although the potential net benefit is uncertain.[63] Critically ill hospitalized patients are recommended to either receive unfractionated heparin or low-molecular weight heparin instead of foregoing these medicines.[63]
After surgery
Major orthopedic surgery—
Options for VTE prevention in people following non-orthopedic surgery include early walking, mechanical prophylaxis, and blood thinners (low-molecular-weight heparin and low-dose-unfractionated heparin) depending upon the risk of VTE, risk of major bleeding, and person's preferences.[159] After low-risk surgeries, early and frequent walking is the best preventive measure.[7]
Pregnancy
The risk of VTE is increased in pregnancy by about four to five times because of a more hypercoagulable state that protects against fatal
Travelers

Travelling "is an often cited yet relatively uncommon" cause of VTE.[28] Suggestions for at-risk[n] long-haul travelers include calf exercises, frequent walking, and aisle seating in airplanes to ease walking.[162][163] Graduated compression stockings have sharply reduced the levels of asymptomatic DVT in airline passengers, but the effect on symptomatic DVT, PE, or mortality is unknown, as none of the individuals studied developed these outcomes.[164] However, graduated compression stockings are not suggested for long-haul travelers (>4 hours) without risk factors for VTE. Likewise, neither aspirin nor anticoagulants are suggested in the general population undertaking long-haul travel.[63] Those with significant VTE risk factors[o] undertaking long-haul travel are suggested to use either graduated compression stockings or LMWH for VTE prevention. If neither of these two methods are feasible, then aspirin is suggested.[63]
Prognosis
DVT is most frequently a disease of older age that occurs in the context of nursing homes, hospitals, and active cancer.[3] It is associated with a 30-day mortality rate of about 6%, with PE being the cause of most of these deaths.[1] Proximal DVT is frequently associated with PE, unlike distal DVT, which is rarely if ever associated with PE.[39] Around 56% of those with proximal DVT also have PE, although a chest CT is not needed simply because of the presence of DVT.[1] If proximal DVT is left untreated, in the following 3 months approximately half of people will experience symptomatic PE.[9]
Another frequent complication of proximal DVT, and the most frequent chronic complication, is post-thrombotic syndrome, where individuals have chronic venous symptoms.
In the 10 years following an initial VTE, about 30% of people will have a recurrence.[3] VTE recurrence in those with prior DVT is more likely to recur as DVT than PE.[167] Cancer[5] and unprovoked DVT are strong risk factors for recurrence.[60] After initial proximal unprovoked DVT with and without PE, 16–17% of people will have recurrent VTE in the 2 years after they complete their course of anticoagulants. VTE recurrence is less common in distal DVT than proximal DVT.[44][45] In upper extremity DVT, annual VTE recurrence is about 2–4%.[130] After surgery, a provoked proximal DVT or PE has an annual recurrence rate of only 0.7%.[60]
Epidemiology
About 1.5 out of 1000 adults a year have a first VTE in high-income countries.[168][169] The condition becomes much more common with age.[3] VTE rarely occurs in children, but when it does, it predominantly affects hospitalized children.[170] Children in North America and the Netherlands have VTE rates that range from 0.07 to 0.49 out of 10,000 children annually.[170] Meanwhile, almost 1% of those aged 85 and above experience VTE each year.[3] About 60% of all VTEs occur in those 70 years of age or older.[9] Incidence is about 18% higher in males than in females,[4] though there are ages when VTE is more prevalent in women.[15] VTE occurs in association with hospitalization or nursing home residence about 60% of the time, active cancer about 20% of the time, and a central venous catheter or transvenous pacemaker about 9% of the time.[3]
During pregnancy and after childbirth, acute VTE occurs in about 1.2 of 1000 deliveries. Despite it being relatively rare, it is a leading cause of maternal morbidity and
DVT occurs in the upper extremities in about 4–10% of cases,[11] with an incidence of 0.4–1.0 people out of 10,000 a year.[5] A minority of upper extremity DVTs are due to Paget–Schroetter syndrome, also called effort thrombosis, which occurs in 1–2 people out of 100,000 a year, usually in athletic males around 30 years of age or in those who do significant amounts of overhead manual labor.[69][147]
Social

Being on blood thinners because of DVT can be life-changing because it can prevent lifestyle activities such as contact or winter sports to prevent bleeding after potential injuries.[175] Head injuries prompting brain bleeds are of particular concern. This has caused NASCAR driver Brian Vickers to forego participation in races. Professional basketball players including NBA players Chris Bosh and hall of famer Hakeem Olajuwon have dealt with recurrent blood clots,[176] and Bosh's career was significantly hampered by DVT and PE.[177]
Tennis star
Other notable people have been affected by DVT. Former United States (US) President
History

The book
In 1856, German physician and pathologist Rudolf Virchow published his analysis after the insertion of foreign bodies into the jugular veins of dogs, which migrated to the pulmonary arteries. These foreign bodies caused pulmonary emboli, and Virchow was focused on explaining their consequences.[189] He cited three factors, which are now understood as hypercoaguability, stasis, and endothelial injury.[190] It was not until 1950 that this framework was cited as Virchow's triad,[189] but the teaching of Virchow's triad has continued in light of its utility as a theoretical framework and as a recognition of the significant progress Virchow made in expanding the understanding of VTE.[189][190]
Methods to observe DVT by ultrasound were established in the 1960s.
Multiple pharmacological therapies for DVT were introduced in the 20th century: oral anticoagulants in the 1940s,
Economics
VTE costs the US healthcare system about $7 to 10 billion dollars annually.[169] Initial and average DVT costs for a hospitalized US patient is about $10,000 (2015 estimate).[196] In Europe, the costs for an initial VTE hospitalization are significantly less, costing about €2000 to 4000 (2011 estimate).[197] Post-thrombotic syndrome is a significant contributor to DVT follow-up costs.[198] Outpatient treatment significantly reduces costs, and treatment costs for PE exceed those of DVT.[199]
Research directions
A 2019 study published in Nature Genetics reported more than doubling the known genetic loci associated with VTE.[14] In their updated 2018 clinical practice guidelines, the American Society of Hematology identified 29 separate research priorities, most of which related to patients who are acutely or critically ill.[63] Inhibition of factor XI, P-selectin, E-selectin, and a reduction in formation of neutrophil extracellular traps are potential therapies that might treat VTE without increasing bleeding risk.[200]
Notes
- retinal vein thrombosis), spleen and intestines (splanchnic vein thrombosis), liver (Budd–Chiari syndrome), kidneys (renal vein thrombosis), and ovaries (ovarian vein thrombosis) are more unusual forms of venous thrombosis and they are considered as separate diseases.[10]
- combined oral contraceptives (COCs) have an approximate two to three times higher risk than second-generation COCs.[64] Progestogen-only pill use is not associated with increased VTE risk.[87]
- ^ Type I[58]
- ^ "It is important to note that smoking is not an independent risk factor, although it increases the risk for cancers and other comorbidities and works synergistically with other independent risk factors."[98]
- ^ The term 'thrombophilia' as used here applies to the five inherited abnormalities of antithrombin, protein C, protein S, factor V, and prothrombin, as is done elsewhere.[89][99] These 5 genetic factors have been referred to as the classical thrombophilias.[100]
- ^ An elevated level is greater than 250 ng/mL D-dimer units (DDU) or greater than 0.5 μg/mL fibrinogen equivalent units (FEU). A normal level is below these values.[113]
- ^ The Wells score as displayed here is the more recent modified score, which added a criterion for a previous documented DVT and increased the time range after surgery to 12 weeks from 4 weeks.[117]
- ^ Subsartorial is a proposed name for a section of the femoral vein.[121]
- NSAIDs.[123]
- ^ The international normalized ratio should be ≥ 2.0 for 24 hours minimum,[18] but if the ratio is > 3.0, then the parenteral anticoagulant is not needed for five days.[126]
- ^ An INR is determined from the ratio of a patient's prothrombin time (PT) to a standardized control PT. A normal INR for those not on anticoagulation is 1.0. A value of 5.0 or higher is considered a critical finding because of an increased risk of bleeding.[127]
- ^ "Up to 83% of patients treated by any catheter-based therapy, need adjunctive angioplasty, and stenting".[5]
- ^ Estimated in United States dollars, estimate published in 2019
- ^ Including those with "previous VTE, recent surgery or trauma, active malignancy, pregnancy, estrogen use, advanced age, limited mobility, severe obesity, or known thrombophilic disorder"[162]
- ^ For example "recent surgery, history of VTE, postpartum women, active malignancy, or ≥2 risk factors, including combinations of the above with hormone replacement therapy, obesity, or pregnancy"[63]
References
- ^ S2CID 173995098.
- ^ S2CID 3707226.
- ^ PMID 26780736.
- ^ PMID 27764878.
- ^ PMID 28329262.
- ^ a b "What is Venous Thromboembolism?". Centers for Disease Control and Prevention. 14 March 2019. Retrieved 6 January 2020.
- ^ PMID 31794602.
- PMID 25304324.
- ^ PMID 29297660. Archived from the originalon 30 January 2020. Retrieved 30 January 2020.
- ^ PMID 31917405.
- ^ PMID 28446351.
- ^ PMID 33007077.
- ^ PMID 31736432.
- ^ PMID 31676865.
- ^ PMID 27126645.
- ^ PMID 29212112.
- ^ PMID 23531017.
- ^ a b c Guyatt et al. 2012, p. 20S: 2.4.
- ^ S2CID 73433650.
- ^ S2CID 34922038.
- ^ PMID 18296592.
- ^ PMID 18031292.
- (PDF) from the original on 6 February 2013.
- S2CID 3454404.
- S2CID 30043830.
- PMID 11762276.
- PMID 31327867.
- ^ PMID 29872243.
- ^ PMID 29101916.
- ^ PMID 31504429.
- S2CID 64951.
- PMID 12997687.
- PMID 29422736.
- ^ Owings JT (2005). "Management of venous thromboembolism". ACS Surgery. American College of Surgeons. Archived from the original on 27 January 2012. Retrieved 16 January 2012.
- PMID 30443615.
- PMID 27113791.
- PMID 32431661.
- PMID 30141306.
- ^ PMID 22315267.
- PMID 18205917.
- PMID 22959541.
- ^ PMID 31258531.
- PMID 28123971.
- ^ PMID 31340984.
- ^ S2CID 242392407.
- S2CID 30043830.
- PMID 20124539.
- PMC 2072980.
- S2CID 33536953.
- PMID 27437827.
- PMID 30586751.
- ^ PMID 31917402.
- PMID 33733032.
- ^ Piazza G (19 October 2019). "Clot Chronicles: unprovoked vs. provoked VTE". North American Thrombosis Forum. Archived from the original on 8 May 2021. Retrieved 8 May 2021.
- ^ S2CID 211037046.
- ^ PMID 22345594.
- PMID 21116184.
- ^ PMID 20456358.
- S2CID 49313388.
- ^ PMID 23531017.
- PMID 21084000.
- ^ S2CID 34486553.
- ^ PMID 30482763.
- ^ S2CID 13564168.
- ^ PMID 19630821.
- S2CID 207452463.
- PMID 27145936.
- PMID 29435334.
- ^ PMID 29399532.
- PMID 25140182.
- PMID 31749809.
- PMID 26111103.
- PMID 33217420.
- PMID 32407386.
- S2CID 220337035.
- S2CID 49606106.
- ^ PMID 29876337.
- PMID 32375490.
- PMID 30472725.
- ^ S2CID 6925903.
- PMID 28331377.
- PMID 31957081.
- PMID 28917273.
- ^ PMID 30918022.
- PMID 29255070.
- ^ PMID 21034220.
- PMID 22872710.
- ^ S2CID 23139811.
- ^ S2CID 9305488.
- ^ S2CID 5203474.
- PMID 31582554.
- PMID 26221164.
- PMID 26179082.
- PMID 25890452.
- PMID 30115029.
- PMID 22076463.
- S2CID 54379879.
- PMID 29262230.
- PMID 22160027.
- PMID 32731838.
- PMID 22082302.
- ^ PMID 25294122.
- PMID 20351121.
- ^ Kim ES, Bartholomew JR. "Venous thromboembolism". Disease Management Project. Cleveland Clinic. Archived from the original on 23 February 2011. Retrieved 15 February 2011.
- ^ PMID 21325673.
- PMID 29034034.
- ^ PMID 32197363.
- PMID 19303501.
- ^ PMID 27354721.
- ^ PMID 31315434.
- S2CID 58594612.
- ^ S2CID 22467822.
- ^ a b "DDI/9290 clinical: D-dimer, plasma". Mayo Medical Laboratories. Archived from the original on 8 October 2012. Retrieved 27 August 2012.
- PMID 23537968.
- PMID 24615063.
- ^ PMID 31845779.
- ^ PMID 29399531.
- ^ S2CID 34220435.
- ^ PMID 26149033.
- ^ PMID 30122174.
- .
- PMID 21422387.
- PMID 16437461.
- PMID 29399516.
- ^ PMID 26867832.
- ^ PMID 22315268.
- PMID 29939529.
- ^ Guyatt et al. 2012, p. 22S: 3.2.
- PMID 25092359.
- ^ PMID 22734929.
- PMID 30482765.
- PMID 21671894.
- S2CID 10659607.
- PMID 30303669.
- PMID 26747198.
- PMID 31573167.
- PMID 29399524.
- ^ a b c d e f g National Institute for Health and Care Excellence. "NICE Guideline 158: Venous thromboembolic diseases: diagnosis, management and thrombophilia testing" London, 26 March 2020.
- S2CID 21801157.
- PMID 26853645.
- ^ PMID 29211671.
- ^ Bhandari T (6 December 2017). "Clot-busting drugs not recommended for most patients with blood clots". Washington University School of Medicine. Retrieved 21 January 2020.
- ^ a b National Institute for Health and Care Excellence. "NICE Interventional procedures guidance 651: Percutaneous mechanical thrombectomy for acute deep vein thrombosis of the leg" London, 12 June 2019.
- PMID 30586751.
- PMID 31592728.
- PMID 31037504.
- ^ PMID 26987584.
- PMID 30646021.
- ^ Moll S (9 May 2012). "What kind of doctor do I need?". Clot Connect. Archived from the original on 28 January 2020. Retrieved 28 January 2020.
- PMID 27429688.
- ^ "Inferior Vena Cava (IVC) Filter Placement". Johns Hopkins Medicine. 2020. Retrieved 28 January 2020.
- PMID 11054217.
- ^ PMID 33191841.
- PMID 25518837.
- S2CID 24036108.
- PMID 23396492.
- S2CID 22797561.
- PMID 22315265.
- PMID 22315263.
- ^ PMID 30482767.
- PMID 22315276.
- ^ PMID 22315261. See section 6.0, Long-Distance Travel
- ^ "New DVT guidelines: No evidence to support "economy-class syndrome"". American College of Chest Physicians. 7 February 2012. Archived from the original on 3 June 2016. Retrieved 10 February 2012.
oral contraceptives, sitting in a window seat, advanced age, and pregnancy increase DVT risk in long-distance travelers
- PMID 33878207.
- PMID 28844444.
- PMID 31899848.
- PMID 25173341.
- PMID 31385593.
- ^ PMID 26654719.
- ^ PMID 30482766.
- PMID 22253396.
- S2CID 17552169.
- ^ "Blood types". American Red Cross. Archived from the original on 14 August 2012. Retrieved 15 August 2012.
- ^ a b Haskell R (10 January 2018). "Serena Williams on motherhood, marriage, and making her comeback". Vogue. Retrieved 22 January 2020.
- S2CID 58564402.
- ^ Perez AJ (17 February 2016). "Expert: Chris Bosh, like many, at risk of blood clot recurrence". USA Today. Retrieved 22 January 2020.
- ^ Martin J (4 May 2016). "Chris Bosh officially out as Heat make playoff push". CNN. Retrieved 22 January 2020.
- ^ Moisse K (2 March 2011). "Serena Williams Hospitalized After Pulmonary Embolism". ABC News. Retrieved 22 January 2020.
- S2CID 231803661.
- PMID 23336651.
- ^ Frankel TC (11 September 2016). "Hillary Clinton has not been quick to share health information". The Washington Post. Retrieved 22 January 2020.
- ^ "Cheney diagnosed with deep-vein thrombosis". The Guardian. 6 March 2007. Retrieved 22 January 2020.
- ^ a b Rodriguez D (12 October 2018). "11 Celebrities Who Battled Deep Vein Thrombosis Risk". Everyday Health. Retrieved 22 January 2020.
- ^ "Coroner: Rapper Heavy D died of blood clot in lung". CNN. 27 December 2011. Retrieved 22 January 2020.
- ^ "My husband should be living today". TODAY. 3 March 2005. Retrieved 22 January 2020.
- ISBN 978-1400052226.
- ^ PMID 24059395.
- PMID 23297815.
- ^ PMID 20739582.
- ^ PMID 18783400.
- ^ Dalen 2003, p. 3.
- PMID 8998158.
- ^ Dalen 2003, p. 2.
- ^ a b Ornstein C, Jones RG (7 January 2015). "The drugs that companies promote to doctors are rarely breakthroughs". The New York Times.
- ^ PMID 27581829.
- PMID 25555319.
- PMID 21222564.
- S2CID 8966676.
- PMID 26355805.
- PMID 30410646.
Cited literature
- Dalen JE (2003). Venous thromboembolism. CRC Press. ISBN 978-0824756451.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ (February 2012). "Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): 7S–47S. PMID 22315257.




![Doppler ultrasonography showing absence of flow and hyperechogenic content in a clotted femoral vein (labeled subsartorial[h]) distal to the branching point of the deep femoral vein. When compared to this clot, clots that instead obstruct the common femoral vein (proximal to this branching point) cause more severe effects due to impacting a significantly larger portion of the leg.[122]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4f/Ultrasonography_of_deep_vein_thrombosis_of_the_femoral_vein_-annotated.jpg/459px-Ultrasonography_of_deep_vein_thrombosis_of_the_femoral_vein_-annotated.jpg)




![After treatment with catheter-directed thrombolysis, blood flow in the axillary and subclavian vein were significantly improved. Afterwards, a first rib resection allowed decompression. This reduces the risk of recurrent DVT and other sequelae from thoracic outlet compression.[147]](http://upload.wikimedia.org/wikipedia/commons/6/6e/A-case-of-Paget-Schroetter-syndrome-%28PSS%29-in-a-young-judo-tutor-a-case-report-13256_2016_848_Fig2_HTML.jpg)