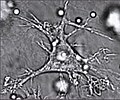
Dendritic cell
| Dendritic cell | |
|---|---|
| Details | |
| System | Immune system |
| Identifiers | |
| Latin | cellula dendritiformis |
| MeSH | D003713 |
| TH | H1.00.01.0.00038 |
| FMA | 83036 |
| Anatomical terminology] | |
A dendritic cell (DC) is an antigen-presenting cell (also known as an accessory cell) of the mammalian immune system. A DC's main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and adaptive immune systems.[1]
Dendritic cells are present in tissues that are in contact with the body's external environment, such as the
History
Dendritic cells were first described by Paul Langerhans (hence Langerhans cells) in the late nineteenth century. The term dendritic cells was coined in 1973 by Ralph M. Steinman and Zanvil A. Cohn.[2] For discovering the central role of dendritic cells in the adaptive immune response,[3] Steinman was awarded the Albert Lasker Award for Basic Medical Research in 2007[4] and the Nobel Prize in Physiology or Medicine in 2011.[5]
Types
The morphology of dendritic cells results in a very large surface-to-volume ratio. That is, the dendritic cell has a very large surface area compared to the overall cell volume.
In vivo – primate
The most common division of dendritic cells is conventional dendritic cells (a.k.a. myeloid dendritic cells) vs. plasmacytoid dendritic cell (most likely of lymphoid lineage) as described in the table below:
| Name | Description | Secretion | Toll-like receptors [6] |
|---|---|---|---|
| Conventional dendritic cell (cDC) (previously called myeloid dendritic cell (mDC)) |
Most similar to monocytes. mDC are made up of at least two subsets:
|
Interleukin 12 (IL-12), Interleukin 6 (IL-6), TNF, chemokines | TLR 4
|
| Plasmacytoid dendritic cell (pDC) | Look like plasma cells, but have certain characteristics similar to myeloid dendritic cells.[7] | Can produce high amounts of interferon-α[8] and were previously called interferon-producing cells.[9] | TLR 9
|
The markers
Lymphoid and myeloid DCs evolve from lymphoid and myeloid precursors, respectively, and thus are of
In blood
The blood DCs are typically identified and enumerated in
In vitro
In some respects, dendritic cells cultured in vitro do not show the same behaviour or capability as dendritic cells isolated ex vivo. Nonetheless, they are often used for research as they are still much more readily available than genuine DCs.
- Mo-DC or MDDC refers to cells matured from monocytes.[12]
- HP-DC refers to cells derived from hematopoietic progenitor cells.
Development and life cycle
Formation of immature cells and their maturation

Dendritic cells are derived from
Every helper T-cell is specific to one particular antigen. Only professional
As mentioned above, mDC probably arise from monocytes, white blood cells which circulate in the body and, depending on the right signal, can turn into either dendritic cells or macrophages. The monocytes in turn are formed from stem cells in the bone marrow. Monocyte-derived dendritic cells can be generated in vitro from peripheral blood mononuclear cell (PBMCs). Plating of PBMCs in a tissue culture flask permits adherence of monocytes. Treatment of these monocytes with interleukin 4 (IL-4) and granulocyte-macrophage colony stimulating factor (GM-CSF) leads to differentiation to immature dendritic cells (iDCs) in about a week. Subsequent treatment with tumor necrosis factor (TNF) further differentiates the iDCs into mature dendritic cells. Monocytes can be induced to differentiate into dendritic cells by a self-peptide Ep1.B derived from apolipoprotein E.[16] These are primarily tolerogenic plasmacytoid dendritic cells.[17]
Life span
In mice, it has been estimated that dendritic cells are replenished from the blood at a rate of 4000 cells per hour, and undergo a limited number of divisions during their residence in the spleen over 10 to 14 days.[18]
Research challenges
The exact genesis and development of the different types and subsets of dendritic cells and their interrelationship is only marginally understood at the moment[when?], as dendritic cells are so rare and difficult to isolate that only in recent years they have become subject of focused research. Distinct surface antigens that characterize dendritic cells have only become known from 2000 on; before that, researchers had to work with a 'cocktail' of several antigens which, used in combination, result in isolation of cells with characteristics unique to DCs.[citation needed]
Cytokines
The dendritic cells are constantly in communication with other cells in the body. This communication can take the form of direct cell–cell contact based on the interaction of cell-surface proteins. An example of this includes the interaction of the membrane proteins of the B7 family of the dendritic cell with CD28 present on the lymphocyte. However, the cell–cell interaction can also take place at a distance via cytokines.[citation needed]
For example, stimulating dendritic cells in vivo with microbial extracts causes the dendritic cells to rapidly begin producing
Disease
Blastic plasmacytoid dendritic cell neoplasm
Blastic plasmacytoid dendritic cell neoplasm is a rare type of
Viral infection
Many other viruses, such as the SARS virus, seem to use DC-SIGN to 'hitchhike' to its target cells.[25] However, most work with virus binding to DC-SIGN expressing cells has been conducted using in vitro derived cells such as moDCs. The physiological role of DC-SIGN in vivo is more difficult to ascertain.
Cancer
Dendritic cells are usually not abundant at tumor sites, but increased densities of populations of dendritic cells have been associated with better clinical outcome, suggesting that these cells can participate in controlling cancer progression.
Autoimmunity
Altered function of dendritic cells is also known to play a major or even key role in allergy and autoimmune diseases like lupus erythematosus and inflammatory bowel diseases (Crohn's disease and ulcerative colitis).[31][32][33]
Other animals
The above applies to humans. In other organisms, the function of dendritic cells can differ slightly. However, the principal function of dendritic cells as known to date is always to act as an immune sentinel. They survey the body and collect information relevant to the immune system, they are then able to instruct and direct the adaptive arms to respond to challenges.
In addition, an immediate precursor to myeloid and lymphoid dendritic cells of the spleen has been identified.[34] This precursor, termed pre-DC, lacks MHC class II surface expression, and is distinct from monocytes, which primarily give rise to DCs in non-lymphoid tissues.
Dendritic cells have also been found in turtles.[35]
Dendritic cells have been found in rainbow trout (Oncorhynchus mykiss) and zebrafish (Danio rerio) but their role is still not fully understood [36]
Media
-
A dendritic cell
-
A well-resolved dendritic cell drags a conidium through a distance of up to 9 μm. The conidium, however, is not phagocytosed by the cell. The observation was made over 3 h with one frame every 30 s.
-
A single dendritic cell can be seen here efficiently taking up at least four conidia in its vicinity.
See also
- Histiocyte
- Macrophage
- List of human clusters of differentiation for a list of CD molecules (such as CD80 and CD86)
- List of distinct cell types in the adult human body
References
- PMID 35265979.
- PMID 4573839.
- S2CID 4388748.
- ^ "The Lasker Foundation – 2007 Awards". Retrieved 27 November 2010.
- ^ "Nobel Prize in Physiology or Medicine for 2011".
- PMID 12110131.
- PMID 15596797.
- PMID 12953097.
- PMID 15771572.
- S2CID 22459468.
- S2CID 1570404.
- PMID 16959355.
- PMID 25578468.
- ^ S2CID 27757632.
- ^ S2CID 5655814.
- S2CID 23966566.
- PMID 24784480.
- S2CID 24736611.
- PMID 9382881.
- PMID 10364556.
- PMID 29760611.
- ^ S2CID 11799428.
- S2CID 3627886.
- PMID 17238285.
- PMID 15140961.
- PMID 25446897.
- PMID 30955881.
- PMID 30979687.
- PMID 27775706.
- PMID 30552023.
- PMID 15647187.
- PMID 19664152.
- S2CID 13544348.
- S2CID 539437.
- PMID 8595821.
- ^ Salinas, I., & Parra, D. (2015). Fish mucosal immunity: Intestine. In Mucosal Health in Aquaculture. Elsevier Inc. https://doi.org/10.1016/B978-0-12-417186-2.00006-6
External links
- [1][dead link] Website of the Center for Infection and Immunity of Lille contains information on DCs and their study in research, link currently dead
- Dendritic+Cells at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- www.dc2007.eu 5th International Meeting on Dendritic Cell Vaccination and other Strategies to tip the Balance of the Immune System
- Website of Ralph M. Steinman at The Rockefeller University Archived 27 June 2009 at the Wayback Machine contains information on DCs, links to articles, pictures and videos
- "Cancer 'danger receptor' found", BBC News, 15 February 2009