Deposition (phase transition)
This article includes a improve this article by introducing more precise citations. (January 2020) ) |

Deposition is the
liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation
and hence sometimes deposition is called desublimation.
Applications
Examples
One example of deposition is the
hoar frost form on the ground or other surfaces. Another example is when frost forms on a leaf. For deposition to occur, thermal energy must be removed from a gas. When the air becomes cold enough, water vapour in the air surrounding the leaf loses enough thermal energy to change into a solid. Even though the air temperature may be below the dew point
, the water vapour may not be able to condense spontaneously if there is no way to remove the latent heat. When the leaf is introduced, the supercooled water vapour immediately begins to condense, but by this point is already past the freezing point. This causes the water vapour to change directly into a solid.
Another example is the soot that is deposited on the walls of chimneys. Soot molecules rise from the fire in a hot and gaseous state. When they come into contact with the walls they cool, and change to the solid state, without formation of the liquid state. The process is made use of industrially in combustion chemical vapour deposition.
Industrial applications
There is an industrial coatings process, known as
thin films
of various materials onto various surfaces.
Deposition releases energy and is an exothermic phase change.
See also
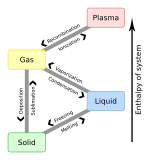
To From
|
Solid | Liquid | Gas | Plasma |
|---|---|---|---|---|
| Solid | Melting | Sublimation | ||
| Liquid | Freezing | Vaporization | ||
| Gas | Deposition | Condensation | Ionization | |
| Plasma | Recombination |
References
- Gaja, Shiv P., Fundamentals of Atmospheric Modeling, Cambridge University Press, 2nd ed., 2005, p. 525 ISBN 978-0-521-83970-9
- Moore, John W., et al., Principles of Chemistry: The Molecular Science, Brooks Cole, 2009, p. 387 ISBN 978-0-495-39079-4
- Whitten, Kenneth W., et al., Chemistry, Brooks-Cole, 9th ed., 2009, p. 7 ISBN 978-0-495-39163-0
- Focus on Physical Science. Glencoe Science.