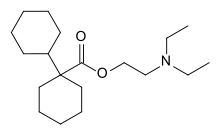
Dicycloverine
 | |
| Clinical data | |
|---|---|
| Trade names | Byclomine, Bentyl, Dibent, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684007 |
| License data |
|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | >99% |
| Elimination half-life | 5 h |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Dicycloverine, also known as dicyclomine, sold under the brand name Bentyl among others, is a
Common side effects include dry mouth, blurry vision, weakness, sleepiness, and lightheadedness.[2] Serious side effects may include psychosis and breathing problems in babies.[2] Use in pregnancy appears to be safe while use during breastfeeding is not recommended.[3] How it works is not entirely clear.[2]
Dicycloverine was approved for medical use in the United States in 1950.
Medical uses
Dicycloverine is used to treat the symptoms of irritable bowel syndrome, specifically hypermotility, in adults.[6][7] As of 2016, clinical guidelines recommended dicycloverine and other antispasmodics for IBS with diarrhea as a first line treatment.[6]
Contraindications
This medicine should not be used for people who have an obstructive GI or urinary condition, severe ulcerative colitis, reflux, any unstable cardiac condition, glaucoma, myasthenia gravis, and anyone who is acutely bleeding.[7]
It should not be given to children or infants with colic due to the risks of convulsions, difficult breathing, irritability, and restlessness,[8] and there is little evidence to support the efficacy in such use in any case.[9]
Dicycloverine is known to impair thinking and coordination.[7]
The effect on the baby during pregnancy or breastfeeding is not well understood.[7]
Adverse effects
Dicycloverine can cause a range of anticholinergic side effects such as dry mouth, nausea, blurred vision, dizziness, confusion, severe constipation, stomach pain, heart palpitations, difficulty urinating, and seizures.[6]
Pharmacology
Dicycloverine blocks the action of
History

Dicycloverine was first synthesized chemically in the United States circa 1945 by scientists at William S. Merrell Company.[10]
It was first marketed in 1952 for gastrointestinal disorders, including colic in infants.
In the 1980s, several governments restricted its use in infants due to reports of convulsions, difficult breathing, irritability, and restlessness in infants given the drug.[8]
In 1994, the US
Society and culture
Rarely, there have been reports of dicycloverine abuse. Dicycloverine is an antagonist at
References
- ^ ISBN 9780857113382.
- ^ a b c d e f g "Dicyclomine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ "Dicyclomine Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Dicyclomine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ PMID 26985553.
- ^ a b c d AHFS Staff (2006). "Dicyclomine hydrochloride". AHFS DI Essentials. Bethesda, MD: American Society of Health-System Pharmacists / drugs.com. Retrieved 25 November 2018.
- ^ a b c Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or Not Approved by Governments (PDF) (12 ed.). World Health Organization. 2005. p. 106. Archived from the original (PDF) on 7 April 2014. Retrieved 25 November 2018.
- PMID 27631535.

- ^ "Dicyclomine". Pubchem. Retrieved 25 November 2018.
- ^ "International Nonproprietary Names for Pharmaceutical Preparations List #3" (PDF). WHO Chronicle. 13 (12): 463–474. December 1959. Retrieved 25 November 2018.
- ^ Sanders J (January 1992). "The Bendectin Litigation: A Case Study in the Life Cycle of Mass Torts" (PDF). Hastings Law Journal. 43 (2): 317. Retrieved 25 November 2018.
- JSTOR 24116860.
- ^ "Bioactivity for AID 625223 - SID 103318016".
- ^ "Bioactivity for AID 625192 - SID 103318016".
- ^ "AID 625151 - DRUGMATRIX: Muscarinic M1 radioligand binding (Ligand: [3H] N-Methylscopolamine) - PubChem".
- ^ "AID 625154 - DRUGMATRIX: Muscarinic M4 radioligand binding (Ligand: [3H] N-Methylscopolamine) - PubChem".
- PMID 24396252.
