Digital polymerase chain reaction
A major contributor to this article appears to have a close connection with its subject. (August 2019) |
Digital polymerase chain reaction (digital PCR, DigitalPCR, dPCR, or dePCR) is a
Principles

The polymerase chain reaction method is used to quantify

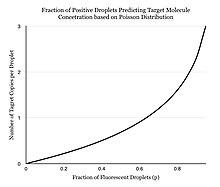
Instead of performing one reaction per well, dPCR involves partitioning the PCR solution into tens of thousands of nano-liter sized droplets, where a separate PCR reaction takes place in each one.[4][5] A PCR solution is made similarly to a TaqMan assay, which consists of template DNA (or RNA), fluorescence-quencher probes, primers, and a PCR master mix, which contains DNA polymerase, dNTPs, MgCl2, and reaction buffers at optimal concentrations. Several different methods can be used to partition samples, including microwell plates, capillaries, oil emulsion, and arrays of miniaturized chambers with nucleic acid binding surfaces.[6] The PCR solution is partitioned into smaller units, each with the necessary components for amplification. The partitioned units are then subjected to thermocycling so that each unit may independently undergo PCR amplification. After multiple PCR amplification cycles, the samples are checked for fluorescence with a binary readout of “0” or “1”. The fraction of fluorescing droplets is recorded.[5] The partitioning of the sample allows one to estimate the number of different molecules by assuming that the molecule population follows the Poisson distribution, thus accounting for the possibility of multiple target molecules inhabiting a single droplet. Using Poisson's law of small numbers, the distribution of target molecule within the sample can be accurately approximated allowing for a quantification of the target strand in the PCR product.[7] This model simply predicts that as the number of samples containing at least one target molecule increases, the probability of the samples containing more than one target molecule increases.[8] In conventional PCR, the number of PCR amplification cycles is proportional to the starting copy number. Different from many people's belief that dPCR provides absolute quantification, digital PCR uses statistical power to provide relative quantification. For example, if Sample A, when assayed in 1 million partitions, gives one positive reaction, it does not mean that the Sample A has one starting molecule.[citation needed]
The benefits of dPCR include increased precision through massive sample partitioning, which ensures reliable measurements in the desired DNA sequence due to reproducibility.[5] Error rates are larger when detecting small-fold change differences with basic PCR, while error rates are smaller with dPCR due to the smaller-fold change differences that can be detected in DNA sequence. The technique itself reduces the use of a larger volume of reagent needed, which inevitably will lower experiment cost. Also, dPCR is highly quantitative as it does not rely on relative fluorescence of the solution to determine the amount of amplified target DNA.
Comparison between dPCR and Real-Time PCR (qPCR)
dPCR measures the actual number of molecules (target DNA) as each molecule is in one droplet, thus making it a discrete “digital” measurement. It provides absolute quantification because dPCR measures the positive fraction of samples, which is the number of droplets that are fluorescing due to proper amplification. This positive fraction accurately indicates the initial amount of template nucleic acid. Similarly, qPCR utilizes fluorescence; however, it measures the intensity of fluorescence at specific times (generally after every amplification cycle) to determine the relative amount of target molecule (DNA), but cannot specify the exact amount without constructing a standard curve using different amounts of a defined standard. It gives the threshold per cycle (CT) and the difference in CT is used to calculate the amount of initial nucleic acid. As such, qPCR is an analog measurement, which may not be as precise due to the extrapolation required to attain a measurement.[6][9]
dPCR measures the amount of DNA after amplification is complete and then determines the fraction of replicates. This is representative of an endpoint measurement as it requires the observation of the data after the experiment is completed. In contrast, qPCR records the relative fluorescence of the DNA at specific points during the amplification process, which requires stops in the experimental process. This “real-time” aspect of qPCR may theoretically affect results due to the stopping of the experiment.[citation needed] In practice, however, most qPCR thermal cyclers read each sample's fluorescence very quickly at the end of the annealing/extension step before proceeding to the next melting step, meaning this hypothetical concern is not actually relevant or applicable for the vast majority of researchers. dPCR measures the amplification by measuring the products of end point PCR cycling and is therefore less susceptible to the artifacts arising from impaired amplification efficiencies due to the presence of PCR inhibitors or primer template mismatch.[10][11]
Real-time Digital PCR (rdPCR) combines the methodologies of digital PCR (dPCR) and quantitative PCR (qPCR), integrating the precision of dPCR with the real-time analysis capabilities of qPCR. This integration aims to provide enhanced sensitivity, specificity, and the ability for absolute quantification of nucleic acid sequences, contributing to the quantification of genetic material in scientific and clinical research.[12][13]
qPCR is unable to distinguish differences in gene expression or copy number variations that are smaller than twofold. On the other hand, dPCR has a higher precision and has been shown to detect differences of less than 30% in gene expression, distinguish between copy number variations that differ by only 1 copy, and identify alleles that occur at frequencies less than 0.1%.[14][5]
Applications
Digital PCR has many applications in
Absolute quantification
dPCR enables the absolute and reproducible quantification of target nucleic acids at single-molecule resolution.
dPCR can quantify, for example, the presence of specific sequences from contaminating
Copy number variation
An alteration in copy number state with respect to a single-copy reference locus is referred to as a “
The large number of “digitized,” endpoint measurements made possible by sample partitioning enables dPCR to resolve small differences in copy number with better accuracy and precision when compared to other methods such as SNP-based microarrays[69] or qPCR.[70][71] qPCR is limited in its ability to precisely quantify gene amplifications in several diseases, including Crohn’s disease, HIV-1 infection, and obesity.[72][68][71]
dPCR was designed to measure the concentration of a nucleic acid target in copies per unit volume of the sample. When operating in dilute reactions where less than ~10% of the partitions contain a desired target (referred to as “limiting dilution”), copy number can be estimated by comparing the number of fluorescent droplets arising from a target CNV with the number of fluorescent droplets arising from an invariant single-copy reference locus.[25] In fact, both at these lower target concentrations and at higher ones where multiple copies of the same target can co-localize to a single partition, Poisson statistics are used to correct for these multiple occupancies to give a more accurate value for each target’s concentration.[73][6]
Digital PCR has been used to uncover both germline and somatic variation in gene copy number between humans
Rare mutation and rare allele detection
Partitioning in digital PCR increases sensitivity and allows for detection of rare events, especially single nucleotide variants (SNVs), by isolating or greatly diminishing the target biomarker signal from potentially competing background.[9][6] These events can be organized into two classes: rare mutation detection and rare sequence detection.
Rare mutation detection
Rare mutation detection occurs when a biomarker exists within a background of a highly abundant counterpart that differs by only a single nucleotide variant (SNV). Digital PCR has been shown to be capable of detecting mutant DNA in the presence of a 200,000-fold excess of wild type background, which is 2,000 times more sensitive than achievable with conventional qPCR.[9]
Rare sequence detection
Digital PCR can detect rare sequences such as HIV DNA in patients with HIV,[24] and DNA from fecal bacteria in ocean and other water samples for assessing water quality.[78] dPCR can detect sequences as rare as 1 in every 1,250,000 cells.[24]
Liquid biopsy
dPCR’s ability to detect rare mutations may be of particular benefit in the clinic through the use of the liquid biopsy, a generally noninvasive strategy for detecting and monitoring disease via bodily fluids.[17][79] Researchers have used liquid biopsy to monitor tumor load, treatment response and disease progression in cancer patients by measuring rare mutations in circulating tumor DNA (ctDNA) in a variety of biological fluids from patients including blood, urine and cerebrospinal fluid.[17][80][81] Early detection of ctDNA (as in molecular relapse) may lead to earlier administration of an immunotherapy or a targeted therapy specific for the patient’s mutation signature, potentially improving chances of the treatment’s effectiveness rather than waiting for clinical relapse before altering treatment. Liquid biopsies can have turnaround times of a few days, compared to two to four weeks or longer for tissue-based tests.[82][83] This reduced time to results has been used by physicians to expedite treatments tailored to biopsy data.[82]
In 2016, a prospective trial using dPCR at the Dana-Farber Cancer Institute authenticated the clinical benefit of liquid biopsy as a predictive diagnostic tool for patients with non-small-cell lung cancer.[84] The application of liquid biopsy tests have also been studied in patients with breast,[85] colorectal,[86][87] gynecologic,[88] and bladder cancers[80][89] to monitor both the disease load and the tumor’s response to treatment.
Gene expression and RNA quantification
Gene expression and RNA quantification studies have benefited from the increased precision and absolute quantification of dPCR.[90] RNA quantification can be accomplished via RT-PCR, wherein RNA is reverse-transcribed into cDNA in the partitioned reaction itself, and the number of RNA molecules originating from each transcript (or allelic transcript) is quantified via dPCR.[31]
One can often achieve greater sensitivity and precision by using dPCR rather than qPCR to quantify RNA molecules in part because it does not require use of a standard curve for quantification.[91] dPCR is also more resilient to PCR inhibitors for the quantification of RNA than qPCR.[47][16][90]
dPCR can detect and quantify more individual target species per detection channel than qPCR by virtue of being able to distinguish targets based on their differential fluorescence amplitude or by the use of distinctive color combinations for their detection.[92][90] As an example of this, a 2-channel dPCR system has been used to detect in a single well the expression of four different splice variants of human telomerase reverse transcriptase, a protein that is more active in most tumor cells than in healthy cells.[93]
Alternative uses for partitioning
Using the dynamic partitioning capabilities employed in dPCR, improved NGS sequencing can be achieved by partitioning of complex PCR reactions prior to amplification to give more uniform amplification across many distinct
Droplet digital PCR
Droplet Digital PCR (ddPCR) is a method of dPCR in which a 20 microliter sample reaction including assay primers and either Taqman probes or an intercalating dye, is divided into ~20,000 nanoliter-sized oil droplets through a water-oil emulsion technique, thermocycled to endpoint in a 96-well PCR plate, and fluorescence amplitude read for all droplets in each sample well in a droplet flow cytometer.[100]
Chip-based digital PCR
Chip-based Digital PCR (dPCR) is also a method of dPCR in which the reaction mix (also when used in qPCR) is divided into ~10,000 to ~45,000 partitions on a chip, then amplified using an endpoint PCR thermocycling machine, and is read using a high-powered camera reader with fluorescence filter (HEX, FAM, Cy5, Cy5.5 and Texas Red) for all partitions on each chip.[101]
History
dPCR rose out of an approach first published in 1988 by Cetus Corporation when researchers showed that a single copy of the β-globin gene could be detected and amplified by PCR.[102][103] This was achieved by diluting DNA samples from a normal human cell line with DNA from a mutant line having a homozygous deletion of the β-globin gene, until it was no longer present in the reaction. In 1989, Peter Simmonds, AJ Brown et al. used this concept to quantify a molecule for the first time.[104] Alex Morley and Pamela Sykes formally established the method as a quantitative technique in 1992.[45]
In 1999, Bert Vogelstein and Kenneth Kinzler coined the term “digital PCR” and showed that the technique could be used to find rare cancer mutations.[105] However, dPCR was difficult to perform; it was labor intensive, required a lot of training to do properly, and was difficult to do in large quantities.[105] In 2003, Kinzler and Vogelstein continued to refine dPCR and created an improved method that they called BEAMing technology, an acronym for “beads, emulsion, amplification and magnetics.” The new protocol used emulsion to compartmentalize amplification reactions in a single tube. This change made it possible for scientists to scale the method to thousands of reactions in a single run.[106][107][108]
Companies developing commercial dPCR systems have integrated technologies like automated partitioning of samples, digital counting of nucleic acid targets, and increasing droplet count that can help the process be more efficient.
References
- PMID 25967899.
- ^ "Polymerase Chain Reaction (PCR)". National Center for Biotechnology Information, U.S. National Library of Medicine.
- S2CID 5714001.
- PMID 29556737.
- ^ S2CID 46347563.
- ^ PMID 29677144.
- ^ Prediger E. "Digital PCR (dPCR)—What is it and why use it?". Integrated DNA Technologies.
- PMID 19698751.
- ^ PMID 21594292.
- PMID 27077029.
- ^ PMID 24003063.
- PMID 26521179.
- PMID 35070745.
- PMID 31704712.
- PMID 27558101.
- ^ PMID 27063479.
- ^ a b c Skibo S (23 Feb 2018). "Has Tumor Profiling Caught Up to Cancer?". Retrieved 23 July 2019.
- ^ Hirsch F (27 July 2018). "Guidelines highlight 'best practices' for liquid biopsy during treatment of non-small cell lung cancer". Retrieved 23 July 2019.
- ^ Johnson M (12 Jan 2018). "Bio-Rad Continues to Advance Digital PCR Tech, Liquid Biopsy Tests Into Commercial Clinical Market". Retrieved 23 July 2019.
- PMID 24429876.
- PMID 28441386.
- ISSN 2383-8442.
- PMID 24406457.
- ^ PMID 23573183.
- ^ )
- PMID 26874951.
- ^ PMID 23637122.
- S2CID 53106591.
- PMID 26957145.
- ^ a b c Marusina K (1 Oct 2017). "Positioning Digital PCR for Sharper Genomic Views". Retrieved 23 July 2019.
- ^ PMID 29717456.
- PMID 29222437.
- ^ "Highly Sensitive Detection of Hepatitis B Using ddPCR". 12 Apr 2018. Retrieved 23 July 2019.
- S2CID 89829687.
- PMID 28626778.
- PMID 26990994.
- PMID 26896631.
- PMID 24509632.
- PMID 26721684.
- PMID 27030102.
- PMID 25979954.
- PMID 27048884.
- PMID 27297323.
- ^ PMID 24781526.
- ^ PMID 1389177.
- PMID 10430926.
- ^ PMID 25628753.
- PMID 29717438.
- PMID 29717439.
- ^ PMID 31549405.
- ^ S2CID 53106591.
- PMID 29238698.
- PMID 29717440.
- PMID 29717441.
- PMID 19208171.
- PMID 25416942.
- ^ Sanders S (16 Jul 2008). "CNVs vs SNPs: Understanding Human Structural Variation in Disease". Retrieved 24 July 2019.
- PMID 27869829.
- PMID 19566914.
- S2CID 149444714.
- ^ "Copy Number Alteration Found to Be Associated with Cancer Mortality". 5 Sep 2018. Retrieved 24 July 2019.
- PMID 19287139.
- PMID 23880486.
- PMID 26814963.
- PMID 25995659.
- PMID 21388883.
- S2CID 8815153.
- ^ PMID 21209899.
- PMID 18682853.
- PMID 28928368.
- ^ PMID 26098870.
- PMID 20858604.
- PMID 29717435.
- PMID 25621458.
- PMID 29717443.
- PMID 27716627.
- PMID 24691421.
- PMID 25543243.
- ^ European Society for Medical Oncology (17 Nov 2017). "Study analyzes mutations in cerebrospinal fluid in lung cancer with brain metastases". Retrieved 24 July 2019.
- ^ a b Petrone J (8 June 2017). "Norwegian Team Plans to Debut Digital PCR-Based Urinary Bladder Cancer Test by Year End". Retrieved 24 July 2019.
- S2CID 4968214.
- ^ a b Paxton A (October 2017). "Revived hopes, fresh challenges with liquid biopsy". Retrieved 24 July 2019.
- ^ Bhadra K, Mellert H, Pestano G (5 Jun 2017). "Adoption of Liquid Biopsy Tests for NSCLC". Retrieved 24 July 2019.
- PMID 27055085.
- PMID 25987569.
- PMID 26451609.
- PMID 25654990.
- PMID 26717006.
- PMID 27417036.
- ^ S2CID 220851187.
- PMID 26315318.
- PMID 27990345.
- PMID 24924392.
- PMID 22426012.
- PMID 26599467.
- S2CID 39450798.
- PMID 29717462.
- S2CID 155519448.
- ^ Stein RA (1 July 2019). "Single-Cell Sequencing Sifts through Multiple Omics". Retrieved 1 August 2019.
- PMID 29717451.
- ^ Low, H., Chan, SJ., Soo, GH. et al. Clarity™ digital PCR system: a novel platform for absolute quantification of nucleic acids. Anal Bioanal Chem 409, 1869–1875 (2017). https://doi.org/10.1007/s00216-016-0131-7
- PMID 2448875.
- PMID 27920991.
- PMID 29378600.
- ^ a b Perkel J (11 April 2014). "The digital PCR revolution". Retrieved 22 July 2019.
- S2CID 28271641.
- PMID 12857956.
- S2CID 7059151.
- ^ Butkus B (8 July 2010). "Digital PCR Space Heating Up as Life Science Tool Vendors Begin Staking Claims". Retrieved 22 July 2019.
- PMID 23329458.
- ^ Butkus B (29 Mar 2012). "RainDance Launches Digital PCR Platform; Claims Sensitivity, Operating Cost Superiority". Retrieved 22 July 2019.
- ^ "'Liquid biopsy' blood test detects genetic mutations in common form of lung cancer". 7 Apr 2016. Retrieved 22 July 2019.
- ^ "Korea's BioCore First to Commercialize NIPT Based on Digital PCR". 2 Mar 2018. Retrieved 22 July 2019.
- ^ "Bio-Rad Gets First CE Mark on Clinical ddPCR Test". 5 Dec 2017. Retrieved 22 Jul 2019.
