Direct factor Xa inhibitors
| Direct factor Xa inhibitor | ||
|---|---|---|
Chemical class Direct factor Xa inhibitors[1] | | |
| Legal status | ||
| In Wikidata | ||
Direct factor Xa inhibitors (xabans) are anticoagulants (blood thinning drugs), used to both treat and prevent blood clots in veins, and prevent stroke and embolism in people with atrial fibrillation (AF).[2][3]
Medical use
Direct factor Xa inhibitors include
Direct factor Xa inhibitors can be considered as an alternative to
Contraindications
Direct Xa inhibitors are contraindicated in people who are actively bleeding or who are at high risk of bleeding. The effects on a fetus or neonate are unknown, hence these drugs are not prescribed in pregnancy or breast feeding mothers.[2]
Adverse effects
Side effects may include
Overdose
A specialist may request a quantitative factor Xa assay in a situation of overdose.[2] Andexanet alfa, a specific antidote to reverse the anticoagulant activity of direct Xa inhibitors in the event of major bleeding, was approved by the FDA in 2018.[9] It is also available in the UK.[10]
Drug interactions
The risk of bleeding is increased if used at the same time as other blood thinning drugs such as
Pharmacology
Mechanism of action
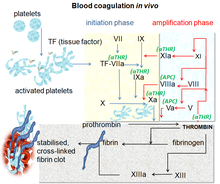
Direct factor Xa inhibitors block the enzyme called
Pharmacokinetics
They have a rapid onset and offset of action. This means it is often possible to pause them 12 to 48 hours before surgery and resume them shortly after the surgery. By contrast, warfarin and phenprocoumon are often paused up to a week before surgery, and low-molecular-weight heparins are used to "bridge" the therapy gap, typically for several weeks.[12][13]
Also in contrast to warfarin and phenprocoumon, direct factor Xa inhibitors do not require frequent monitoring of the prothrombin time (also called the INR) and dose adjustments.[12]
History

Prior to the introduction of direct factor Xa inhibitors,
A naturally occurring inhibitor of factor Xa was reported in 1971 by Spellman et al. from the dog hookworm.[15] In 1987, Tuszynski et al. discovered antistasin, which was isolated from the extracts of the Mexican leech, Haementeria officinalis.[16][17] Later, another naturally occurring inhibitor, tick anticoagulant peptide (TAP) was isolated from the extract of tick Ornithodoros moubata.[18] Trials subsequently demonstrated efficacy and safety against warfarin for stroke prevention in AF and against LMWH for treatment and prevention of VTE including in people undergoing hip or knee replacement.[19]
Society and culture
Economics
The cost of direct Xa inhibitors can reach more than 50 times that of warfarin, although this difference may be offset by lower monitoring costs.[2][5]
Brand names
Brands include
Discontinued xabans
Xabans that never reached the market include darexaban (YM150) from Astellas,[22][23] otamixaban from Sanofi,[24] letaxaban (TAK-442) from Takeda,[25] and eribaxaban (PD0348292) from Pfizer.[26]
References
- ^ "WHOCC – ATC/DDD Index". whocc.no. Archived from the original on 4 April 2021. Retrieved 4 April 2021.
- ^ ISBN 978-0-7020-7442-4.
- ^ ISBN 978-1-284-29449-1.
- ^ "List of Factor Xa inhibitors". Drugs.com. Archived from the original on 4 April 2021. Retrieved 4 April 2021.
- ^ ISBN 978-0-85711-369-6.
- ^ a b American Society of Hematology (2008). "Antithrombotic Therapy - Hematology.org". hematology.org. Archived from the original on 8 April 2021. Retrieved 8 April 2021.
- PMID 32538234.
- ^ ISBN 9783319546438.
- ^ Portola Pharmaceuticals, Inc. "U.S. FDA Approves Portola Pharmaceuticals' Andexxa, First and Only Antidote for the Reversal of Factor Xa Inhibitors". GlobeNewswire News Room. Retrieved 5 April 2021.
- ISBN 978-0-85711-369-6.
- PMID 29636974.
- ^ a b Haberfeld H, ed. (2020). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Xarelto 20 mg Filmtabletten; Eliquis 5 mg Filmtabletten; Lixiana 60 mg Filmtabletten.
- PMID 25472710.
- PMID 26710352.
- PMID 5102508.
- PMID 2745433.
- ^ "P15358 antistasin". Uniprot. Retrieved 11 April 2014.
- ISBN 978-1-59259-658-4.
- PMID 24319220.
- PMID 18096568.
- S2CID 35948814.
- ^ Grogan, Kevin (29 September 2011). "Astellas pulls the plug on darexaban". Pharmatimes. Archived from the original on 13 April 2014. Retrieved 11 April 2014.
- ^ "Astellas Pharma Inc. Discontinues Development of Darexaban (YM150),an Oral Direct Factor Xa Inhibitor". Astellas Pharma. 28 September 2011.
- ^ "AstraZeneca, Sanofi Cut Programs". Chemical & Engineering News. 91 (23). American Chemical Society: 17. 10 June 2013.
Sanofi is ending development on two compounds, the anticancer compound iniparib and the anticoagulant otamixaban, both of which flunked Phase III studies.
- ^ Dwyer J, Walsh C (May 2013). "First Time European Approval for Xarelto in ACS". Decision Resources. Archived from the original on 19 July 2014.
- ^ "Eribaxaban". AdisInsight. 14 March 2021.
