Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors
Discovery and development of
History
In the summer of 1981 the acquired immunodeficiency syndrome (AIDS) was first reported.
The HIV-1 reverse transcriptase enzyme
Function

Most standard HIV drug therapies revolve around inhibiting the reverse transcriptase enzyme (RT), an enzyme that is necessary to the HIV-1 virus and other
Structure
HIV-1 RT is an asymmetric heterodimer which is 1000
Mechanism of action
Activation of nucleoside and nucleotide reverse-transcriptase inhibitors is primarily dependent on cellular entry by
Nucleosides must be triphosphorylated, while nucleotides, which possess one phosphonated group, must be diphosphorylated.
Discovery and development
First step towards treatment of HIV- zidovudine
In 1964 zidovudine (AZT) was synthesized by Horwitz at the Michigan Cancer Foundation. The 3´hydroxyl group in the deoxyribose ring of thymidine is replaced by an
Further development of nucleoside analogues
Dideoxynucleosides
| Dideoxyadenosine | Didanosine | |
|---|---|---|
| Chemical
structure |

|

|
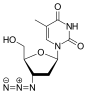
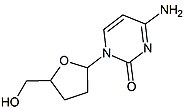
Dideoxynucleosides are analogues of nucleoside where the sugar ring lacks both 2´ and 3´-hydroxyl groups.[9] Three years after the synthesis of zidovudine, Jerome Horwitz and his colleagues in Chicago prepared another dideoxynucleoside now known as zalcitabine (ddC).[16] Zalcitabine is a synthetic pyrimidine nucleoside analogue, structurally related to deoxycytidine, in which the 3´-hydroxyl group of the ribose sugar moiety is substituted with hydrogen.[17] Zalcitabine was approved by the FDA for the treatment of HIV-1 in June 1992.[3][18]
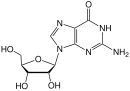
2´,3´-dideoxyinosine or didanosine is converted into dideoxyadenosine in vivo. Its development has a long history.[19] In 1964 dideoxyadenosine, the corresponding adenosine analogue of zalcitabine was synthesised. Dideoxyadenosine caused kidney damage so didanosine was prepared from dideoxyadenosine by enzymatic oxidation (see table 1). It was found to be active against HIV without causing kidney damage.[16] Didanosine was approved by the FDA for the treatment of HIV-1 in October 1991.[18] Zalcitabine and didanosine are both obligate chain terminators, that have been developed for anti-HIV treatment. Unfortunately, both drugs lack
| Zalcitabine | Lamivudine | |
|---|---|---|
| Chemical
structure |
|

|
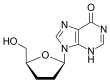
Further modification of the dideoxy framework led to the development of 2´,3´-didehydro-3´-deoxythymidine (stavudine, d4T). Activity of stavudine was shown to be similar to that of zidovudine, although their phosphorylation patterns differ; the
for stavudine is 700-fold weaker.[9]
2',3'-dideoxy-3'-thiacytidine (lamivudine, 3TC) was discovered by Bernard Belleau. The history
of lamivudine can be traced back to the mid-1970s while Bernard Belleau was investigating sugar
Next in line was 2',3'-dideoxy-5-fluoro-3'-thiacytidine (Emtricitabine, FTC) which is a structural homologue of lamivudine. The structural difference is the 5-fluoro-modification of the base moiety of lamivudine. It is similar in many ways to lamivudine and is active against both HIV-1 and hepatitis B virus (HBV).[21][22]
Carbocyclic nucleoside
Carbocyclic analogues of dideoxyadenosine were investigated for their anti-HIV activity. Minimal activity was first observed. Many nucleoside analogues were prepared and examined but only one had significant activity and satisfied the requirements for
This drug is the only approved antiretroviral that is active as a guanosine analogue in vivo. First it is monophosphorylated by adenosine phosphotransferase and then the monophosphate is converted to carbovir 3´-monophosphate. Subsequently, it is fully phosphorylated and the carbovir is incorporated by the RT into the DNA chain and acts as a chain terminator. Carbovir is a related guanosine analogue that had poor oral bioavailability and thus was withdrawn from clinical development.[19]
| Dideoxyadenosine | Didanosine | Abacavir | |
|---|---|---|---|
| Chemical structure | 
|

|

|
Acyclic nucleotide – the only approved NtRTI
Nucleotide analogues require only two phosphorylation steps whereas nucleoside analogues require three steps. Reduction in the phosphorylation requirement may allow more rapid and complete conversion of drugs to their active metabolites. Such considerations have led to the development of phosphonate nucleotide analogues such as tenofovir. Tenofovir disoproxil fumarate (Tenofovir DF) is the
why does the table eat the next section heading if nothing is written here?
Resistance
Currently, appearance of
Two main mechanisms are known that cause NRTI drug resistance: Interference with the incorporation of NRTIs and excision of incorporated NRTIs.
Current status
Currently, there are several NRTIs in various stages of clinical and
Apricitabine (ATC)
Apricitabine is a deoxycytidine analogue. It is structurally related to lamivudine where the positions of the oxygen and the sulfur are essentially reversed.[21] Even though apricitabine is a little less potent in vitro compared to some other NRTIs, it maintains its activity against a broad spectrum of HIV-1 variants with NRTI resistance mutations. Apricitabine is in the final stage of clinical development for the treatment of NRTI-experienced patients.[6]
Elvucitabine (L-d4FC)
Amdoxovir (DAPD)
Amdoxovir is a guanosine analogue NRTI prodrug that has good bioavailability.[6][22][29] It is deaminated intracellularly by adenosine deaminase to dioxolane guanine (DXG). DXG-triphosphate, the active form of the drug, has greater activity than DAPD-triphosphate.[22] Amdoxovir is currently in phasa II clinical trials.[24][29]
Racivir (RCV)
Racivir is a racemic mixture of the two β-enantiomers of emtricitabine (FTC), (-)-FTC and (+)-FTC. Racivir has excellent oral bioavailability and has the advantage of needing to be taken only once a day. Racivir can be considered to be used in combination of two NRTIs and has shown promising antiviral activity when used in combination. Racivir is currently in phase II clinical trials.[6][22][29]
There are several more NRTIs in development. Either the sponsors have filed for an Investigational New Drug (IND) application, the application has been approved by the FDA or the drugs are in different phases of clinical trials. Some of the NRTIs that are in development exhibit various attractive pharmacological properties that could make them desirable for the treatment of patients in need of new agents.[6][22][29]
See also
- Antiretroviral drug
- Discovery and development of CCR5 receptor antagonists
- Discovery and Development of Non-Nucleoside Reverse-Transcriptase Inhibitors
- Discovery and development of HIV protease inhibitors
- Discovery and development of CCR5 receptor antagonists
- HIV/AIDS research
- Reverse-transcriptase inhibitor
- Protease inhibitor
- Entry inhibitor
References
- S2CID 2579436
- PMID 10502595
- ^ ProQuest 223114463
- ^ PMID 19108994
- ^ PMID 18667707
- ^ PMID 19887088
- ^ PMID 18291546
- ^ PMID 19632850
- ^ S2CID 24960391
- ^ PMID 15183338
- PMID 10929917
- S2CID 6954555
- ^ ISBN 978-0-471-94847-6
- ^ ISBN 978-90-5702-037-7
- ^ a b Saunders, J. (2000), Top drugs: Top synthetic routes, pp. 71–75
- ^ ISBN 978-0-471-89979-2
- ISBN 978-1-60327-296-4)
{{citation}}: CS1 maint: DOI inactive as of January 2024 (link - ^ PMID 19108994
- ^ ISBN 978-0-07-142280-2
- ^ ISBN 978-1-58829-649-8
- ^ a b c d LaFemina, R.L. (2009), Antiviral research strategies in antiviral drug discovery, AMS press, pp. 51–70
- ^ PMID 15351346
- ^ PMID 12462284
- ^ PMID 11018598
- PMID 15572156, archived from the original(PDF) on 2006-01-30, retrieved 2010-11-03
- ^ PMID 21088701
- ^ PMID 20376302
- PMID 10508010
- ^ a b c d e f Agrawala, R.K.; Krishnan, P.N.; Raman, S.; Ravichandran, S.; Veerasamy, R. (2008), "An overview on HIV-1 reverse transcriptase inhibitors" (PDF), Digest Journal of Nanomaterials and Biostructures, 3 (4): 171–187, archived from the original (PDF) on 2011-07-20