Enantiomer
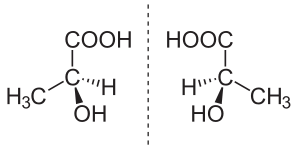
In
A molecule with chirality rotates plane-polarized light.[6] A mixture of equal amounts of each enantiomer, a racemic mixture or a racemate, does not rotate light.[7][8] [9]
Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are also nonsuperposable onto each other; however, they are not mirror images of each other.[10]
Naming conventions
There are three common naming conventions for specifying one of the two enantiomers (the absolute configuration) of a given chiral molecule: the R/S system is based on the geometry of the molecule; the (+)- and (−)- system (also written using the obsolete equivalents d- and l-) is based on its optical rotation properties; and the D/L system is based on the molecule's relationship to enantiomers of glyceraldehyde.
The R/S system is based on the molecule's geometry with respect to a chiral center.[11] The R/S system is assigned to a molecule based on the priority rules assigned by Cahn–Ingold–Prelog priority rules, in which the group or atom with the largest atomic number is assigned the highest priority and the group or atom with the smallest atomic number is assigned the lowest priority.
The (+)- and (−)- is used to specify a molecule's optical rotation — the direction that the molecule rotates in polarized light.[12] When a molecule is denoted dextrorotatory it is rotating the plane of polarized light clockwise and can also be denoted as (+).[11] When it is denoted as levorotatory it is rotating the plane of polarized light counterclockwise and can also be denoted as (−).[11]
The Latin words for left are laevus and sinister, and the word for right is dexter (or rectus in the sense of correct or virtuous). The English word right is a
The prefix ar-, from the Latin recto (right), is applied to the right-handed version; es-, from the Latin sinister (left), to the left-handed molecule.[citation needed] Example: ketamine, arketamine, esketamine.
Chirality centers
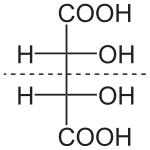
The asymmetric atom is called a chirality center,
Compounds that contain exactly one (or any odd number) of asymmetric atoms are always chiral. However, compounds that contain an even number of asymmetric atoms sometimes lack chirality because they are arranged in mirror-symmetric pairs, and are known as
Even though a chiral molecule lacks reflection (Cs) and rotoreflection symmetries (S2n), it can have other molecular symmetries, and its symmetry is described by one of the chiral point groups: Cn, Dn, T, O, or I. For example, hydrogen peroxide is chiral and has C2 (two-fold rotational) symmetry. A common chiral case is the point group C1, meaning no symmetries, which is the case for lactic acid.
Examples
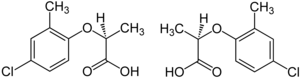

An example of such an enantiomer is the sedative thalidomide, which was sold in a number of countries around the world from 1957 until 1961. It was withdrawn from the market when it was found to cause birth defects. One enantiomer caused the desirable sedative effects, while the other, unavoidably[22] present in equal quantities, caused birth defects.[23]
The herbicide mecoprop is a racemic mixture, with the (R)-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.[24]
Another example is the antidepressant drugs
Chiral drugs
Enantiopure compounds consist of only one of the two enantiomers. Enantiopurity is of practical importance since such compositions have improved therapeutic efficacy.
Enantioselective preparations
In the absence of an effective enantiomeric environment (precursor, chiral catalyst, or kinetic resolution), separation of a racemic mixture into its enantiomeric components is impossible, although certain racemic mixtures spontaneously crystallize in the form of a racemic conglomerate, in which crystals of the enantiomers are physically segregated and may be separated mechanically. However, most racemates form crystals containing both enantiomers in a 1:1 ratio.
In his pioneering work, Louis Pasteur was able to isolate the isomers of sodium ammonium tartrate because the individual enantiomers crystallize separately from solution. To be sure, equal amounts of the enantiomorphic crystals are produced, but the two kinds of crystals can be separated with tweezers. This behavior is unusual. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high
A third strategy is Enantioconvergent synthesis, the synthesis of one enantiomer from a racemic precursor, utilizing both enantiomers. By making use of a chiral catalyist, both enantiomers of the reactant result in a single enantiomer of product.[30]
Enantiomers may not be isolable if there is an accessible pathway for racemization (interconversion between enantiomorphs to yield a racemic mixture) at a given temperature and timescale. For example, amines with three distinct substituents are chiral, but with few exceptions (e.g. substituted N-chloroaziridines), they rapidly undergo "
Parity violation
For all intents and purposes, each enantiomer in a pair has the same energy. However, theoretical physics predicts that due to
Quasi-enantiomers
Quasi-enantiomers are molecular species that are not strictly enantiomers, but behave as if they are. In quasi-enantiomers majority of the molecule is reflected; however, an atom or group within the molecule is changed to a similar atom or group.[33] Quasi-enantiomers can also be defined as molecules that have the potential to become enantiomers if an atom or group in the molecule is replaced.[34] An example of quasi-enantiomers would (S)-bromobutane and (R)-iodobutane. Under normal conditions the enantiomers for (S)-bromobutane and (R)-iodobutane would (R)-bromobutane and (S)-iodobutane respectively. Quasi-enantiomers would also produce quasi-racemates, which are similar to normal racemates (see Racemic mixture) in that they form an equal mixture of quasi-enantiomers.[33]
Though not considered actual enantiomers, the naming convention for quasi-enantiomers also follows the same trend as enantiomers when looking at (R) and (S) configurations - which are considered from a geometrical basis (see Cahn–Ingold–Prelog priority rules).
Quasi-enantiomers have applications in parallel kinetic resolution.[35]
See also
References
- ^ "Compare Synonyms: See How The Synonyms Differ". Thesaurus.com. Retrieved 2022-11-17.
- . Retrieved 2022-11-17.
- . Retrieved 2022-11-17.
- . Retrieved 2022-11-17.
- PMID 15156233.
- ^ "Chirality and Optical Activity". chemed.chem.purdue.edu. Retrieved 2022-11-17.
- . Retrieved 2022-11-17.
- . Retrieved 2022-11-17.
- ^ Weber, Erin. "Library Guides: CHEM 221: Stereochemistry / Isomerism". libraryguides.salisbury.edu. Retrieved 2022-11-17.
- ISBN 978-0-471-72091-1
- ^ ISSN 0022-3263.
- S2CID 12367578.
- ^ ISSN 0021-9584.
- ^ a b c Karras, Manfred (2018). "Synthesis of Enantiomerically Pure Helical Aromatics Such As NHC Ligands and Their Use in Asymmetric Catalysis (PhD). Charles University. Retrieved 6 August 2021.
- ^ OCLC 27642721.
- ^ OCLC 761379371.
- OCLC 1180250839.)
{{cite book}}: CS1 maint: location missing publisher (link - ISSN 0002-7863.
- .
- ISBN 0-471-21495-7.
- .
- S2CID 36115871.
- S2CID 33537650.
- ^ Ariens, E.J (1989). Chiral Separations by HPLC. Chichester: Ellis Horwwod. pp. 31–68.
- ^ "European Medicines Agency - - Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone)". www.ema.europa.eu. 17 September 2018. Archived from the original on 1 December 2017. Retrieved 14 February 2011.
- ISBN 0-520-23945-8.
- PMID 27829909. Retrieved 4 August 2021.
- OCLC 319518566.
- S2CID 231879820.
- ^ PMID 12010052.
- PMID 15915521.
- ^ G.S. Coumbarides, M. Dingjan, J. Eames, A. Flinn, J. Northen and Y. Yohannes, Tetrahedron Lett. 46 (2005), p. 2897er
External links
 Media related to Enantiomers at Wikimedia Commons
Media related to Enantiomers at Wikimedia Commons- chemwiki:stereoisomerism
