Shigatoxigenic and verotoxigenic Escherichia coli
| Shigatoxigenic and verotoxigenic E. coli | |
|---|---|
Infectious disease | |
Shigatoxigenic Escherichia coli (STEC) and verotoxigenic E. coli (VTEC) are strains of the bacterium
Background
The best known of these strains is
EHECs that induce bloody diarrhea lead to HUS in 10% of cases. The clinical manifestations of postdiarrheal HUS include acute renal failure, microangiopathic hemolytic anemia, and thrombocytopenia. The verocytotoxin (shiga-like toxin) can directly damage renal and endothelial cells. Thrombocytopenia occurs as platelets are consumed by clotting. Hemolytic anemia results from intravascular fibrin deposition, increased fragility of red blood cells, and fragmentation.[6]
Antibiotics are of questionable value and have not shown to be of clear clinical benefit. Antibiotics that interfere with DNA synthesis, such as
The clinical presentation ranges from a mild and uncomplicated
Names
Names of the group and its subgroups include the following.
The current microbiology-based view on "Shiga-like toxin" (SLT) or "verotoxin" is that they should all be referred to as (versions of) Shiga toxin, as the difference is negligible. Following this view, all "VTEC" (blue) should be called "STEC" (red).[1][10]: 2–3 Historically, a different name was sometimes used because the toxins are not exactly the same as the one found in Shigella dysenteriae, down to every last amino acid residue, although by this logic every "STEC" would be a "VTEC". The line can also be drawn to use "STEC" for Stx1-producing strains and "VTEC" for Stx2-producing strains, since Stx1 is closer to the Shiga toxin. Practically, the choice of words and categories is not as important as the understanding of clinical relevance.
| Name | Short form |
|---|---|
| enterohemorrhagic E. coli | EHEC |
| hemolytic uremic syndrome–associated enterohemorrhagic E. coli | HUSEC |
| shiga toxin–producing E. coli | STEC |
| shigatoxigenic E. coli | STEC |
| shiga-like toxin–producing E. coli | SLTEC |
| verotoxin-producing E. coli | VTEC |
| verotoxigenic E. coli | VTEC |
| verocytotoxin-producing E. coli | VTEC |
| verocytotoxigenic E. coli | VTEC |
Infectivity and virulence
PMID 19239748.. . (February 2021) |
The infectivity or the virulence of an EHEC strain depends on several factors, including the presence of fucose in the medium, the sensing of this sugar and the activation of EHEC pathogenicity island.[citation needed]

Regulation of the pathogenicity island
This article may be too technical for most readers to understand. (December 2015) |
EHEC becomes pathogenic through the expression of the locus of enterocyte effacement (LEE) encoded on its pathogenicity island. However, when EHEC is not in a host this expression is a waste of energy and resources, so it is only activated if some molecules are sensed in the environment. [citation needed]
When QseC or QseE bind with one of their interacting signalling molecule, they autophosphorylate and transfer its phosphate to the response regulator. QseC senses
As a result, QseC phosphorylates QseB (which activates flagella), KpdE (which activates the LEE) and QseF. QseE phosphorylates QseF. The products QseBC and QseEF repress the expression of FusK and FusR. FusK and FusR are the two components of a system to repress the transcription of the LEE genes. FusK is a sensor kinase which is able to sense many sugars among which fucose. When fucose is present in the medium FusK phosphorylates FusR which represses LEE expression. [citation needed]
Thus when EHEC enters the gut there is a competition between the signals coming from QseC and QseF, and the signal coming from FusK. The first two would like to activate virulence, but Fusk stops it because the mucous layer, which is a source of fucose, isolates enterocytes from bacteria making the synthesis of the virulence factors useless. However, when fucose concentration decreases because bacterial cells find an unprotected area of the epithelium, then the expression of LEE genes will not be repressed by FusR, and KpdE will strongly activate them. In summary, the combined effect of the QseC/QseF and FusKR provide a fine-tuning system of LEE expression which saves energy and allow the mechanisms of virulence to be expressed only when the chances of success are higher.[citation needed]
Shiga toxins
Shiga toxins are a major virulence factor of EHEC. The toxins interact with intestinal epithelium and can cause systematic complications in humans like HUS and cerebral dysfunction if they enter the circulation.[11] In EHEC, Shiga toxins are encoded lysogenic bacteriophages. The toxins bind to cell-surface glycolipid receptor Gb3, which causes the cell to take the toxin in via endocytosis. The Shiga toxins target ribosomal RNA, which inhibits protein synthesis and causes apoptosis.[12] The reason EHEC are symptomless in cattle is because the cattle do not have vascular expression of Gb3 unlike humans. Thus, the Shiga toxins cannot pass through the intestinal epithelium into circulation.[5]
FusKR complex
This section may require cleanup to meet Wikipedia's quality standards. The specific problem is: do we really need the ORF numbers? (February 2021) |
This complex, formed by two components (FusK and FusR) has the function in EHEC to detect the presence of fucose in the environment and regulate the activation of LEE genes.[citation needed]
- FusK: is encoded by the z0462 gene. This gene is an histidine kinase sensor. It detects fucose and then phosphorylates the Z0463 gene activating it.
- FusR: is encoded by the z0463 gene. This gene is a repressor of LEE genes. When z0462 gene detects fucose, phosphorylates and activates the Z0463 gene, which will repress the expression of 'le r', the regulator of the LEE genes. If z0463 gene is not active, the expression of the gene ler would not be repressed. The expression of 'ler' activates the remaining genes in the pathogenicity island inducing virulence.
- At the same time, the system FusKR inhibits the Z0461 gene, a fucose transporter.[citation needed]

Fucose increases the activation of the FusKR system, which inhibits the z0461 gene, which controls the metabolism of fucose. This is a mechanisms that is useful to avoid the competition for fucose with other strains of E. coli which are usually more efficient at using fucose as a carbon source. High concentrations of fucose in the medium also increases the repression of the LEE genes.
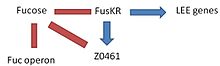
With low levels of fucose in the environment, the FusKR system is inactive, and this means that z0461 gene is transcribed, thus increasing the metabolism of fucose. Furthermore, a low concentration of fucose is an indication of unprotected epithelium, thus the repression of ler genes will disappear and the expression of the LEE genes will allow to attack the adjacent cells.[citation needed]
See also
Notes
References
- ^ PMID 22760050.
- PMID 24092857.
- ^ PMID 10367357.
- PMID 10940275.
- ^ PMID 10973498.
- ^ PMID 16807997. Two sentences were taken from this source verbatim.
- ^ a b Mallove, Zach (26 April 2010). "Lawyer Battles FSIS on Non-O157 E. coli". Food Safety News. Retrieved 2 June 2011.
- PMID 10669353.
- PMID 16238016.
- ISBN 978-3319505800.
- PMID 32512916.
- PMID 12398208.
Further reading
- Bardiau, M.; M. Szalo & J.G. Mainil (2010). "Initial adherence of EPEC, EHEC and VTEC to host cells". Vet Res. 41 (5): 57. PMID 20423697.
- Wong, A.R.; et al. (2011). "Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements". Mol Microbiol. 80 (6): 1420–38. S2CID 24606261.
- Tatsuno, I. (2007). "[Adherence of enterohemorrhagic Escherichia coli O157:H7 to human epithelial cells]". Nihon Saikingaku Zasshi. 62 (2): 247–53. PMID 17575791.
- Kaper, J.B.; J.P. Nataro & H.L. Mobley (2004). "Pathogenic Escherichia coli". Nat Rev Microbiol. 2 (2): 123–40. S2CID 3343088.
- Garcia, A.; J.G. Fox & T.E. Besser (2010). "Zoonotic enterohemorrhagic Escherichia coli: A One Health perspective". ILAR J. 51 (3): 221–32. PMID 21131723.
- Shimizu, T. (2010). "[Expression and extracellular release of Shiga toxin in enterohemorrahgic Escherichia coli]". Nihon Saikingaku Zasshi. 65 (2–4): 297–308. PMID 20505269.
