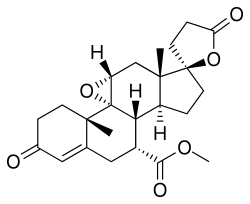
Eplerenone
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛpˈlɛrənoʊn/ |
| Trade names | Inspra, Epnone, Dosterep |
| Other names | SC-66110; CGP-30083; 9-11α-Epoxymexrenone; 9,11α-Epoxy-7α-methoxycarbonyl-3-oxo-17α-pregn-4-ene-21,17-carbolactone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603004 |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~70%[2] |
| Protein binding | ~50% (33–60%) (primarily to α1-acid glycoprotein)[2][3] |
| Metabolism | Liver (CYP3A4)[2][3] |
| Metabolites | 6β-OH-EPL, 6β,21-OH-EPL, 21-OH-EPL, 3α,6β-OH-EPL[2] (All inactive)[2] |
| Elimination half-life | 4–6 hours[4] |
| Excretion | Urine (67%), feces (32%)[5] |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Eplerenone, sold under the brand name Inspra, is an
Medical uses
Heart failure
Eplerenone reduces risk of death in patients with heart failure,[7] particularly in patients with recent myocardial infarction (heart attack).[8]
Hypertension
Eplerenone lowers blood pressure in patients with primary hypertension.[9] Eplerenone also reduces arterial stiffness and vascular endothelial dysfunction.[10]
For persons with resistant hypertension, eplerenone is safe and effective for reducing blood pressure,[11] particularly in persons with resistant hypertension due to hyperaldosteronism.[12][13]
Central serous chorioretinopathy
Eplerenone is often prescribed for people with central serous chorioretinopathy (CSC). However, the most recent and largest randomized controlled trial showed that eplerenone has no significant effect on chronic CSC that has been untreated for four months.[14][15]
Adverse effects
Common
Currently, there is not enough evidence available from the randomized controlled trials on side effects of eplerenone to do a benefit versus risk assessment in people with primary hypertension.[18]
Interactions
Eplerenone is primarily metabolized by the
Pharmacology
Eplerenone is an
Eplerenone seems to be about 50 to 75% as potent as spironolactone as an antimineralocorticoid.[23] Hence, 25 mg/day spironolactone may be equivalent to approximately 50 mg/day eplerenone.[24]
Regulatory and Patent History
Eplerenone was patented in 1983 and approved for medical use in the United States in 2002.[25][22] Eplerenone is currently approved for sale in Canada, the US, EU, Netherlands and Japan.[22] Eplerenone costs an estimated $2.93 per day when treating congestive heart failure and $5.86 per day when treating hypertension.[17]
See also
References
- FDA. Retrieved 22 Oct 2023.
- ^ ISBN 978-1-60913-345-0.
- ^ S2CID 21437788.
- ^ PMID 18404673.
- ISBN 978-1-57340-221-7.
- PMID 11607037.
- S2CID 198999728.
- S2CID 219936888.
- PMID 28245343.
- PMID 33461316.
- PMID 25801902.
- S2CID 218670505.
- PMID 28449833.
- S2CID 241440498.
- PMID 31982075.
- ISBN 978-0-9757919-2-9.
- ^ PMID 16200104.
- PMID 28245343.
- ^ LoSalt Advisory Statement Archived 2005-12-10 at the Wayback Machine (PDF)
- ^ PMID 10760075.
- S2CID 13904574.
- ^ a b c "Inspra (Eplerenone)". Drug Development Technology. Retrieved 2016-04-19.
- PMID 18404673.
- ISBN 978-0-7295-7927-8.
- ISBN 9783527607495.
