Epoxide

In
Nomenclature
A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound 1,2-epoxyheptane, which can also be called 1,2-heptene oxide.
A polymer formed from epoxide precursors is called an epoxy, but such materials do not contain epoxide groups (or contain only a few residual epoxy groups that remain unreacted in the formation of the resin).
Synthesis
The dominant epoxides industrially are ethylene oxide and propylene oxide, which are produced respectively on the scales of approximately 15 and 3 million tonnes/year.[2]
Heterogeneously catalyzed oxidation of alkenes
The epoxidation of ethylene involves its reaction with oxygen. According to a reaction mechanism suggested in 1974[3] at least one ethylene molecule is totally oxidized for every six that are converted to ethylene oxide:
The direct reaction of oxygen with alkenes is useful only for this epoxide. Modified heterogeneous silver catalysts are typically employed.[4] Other alkenes fail to react usefully, even propylene, though TS-1 supported Au catalysts can perform propylene epoxidation selectively.[5]
Olefin (alkene) oxidation using organic peroxides and metal catalysts
Aside from ethylene oxide, most epoxides are generated by treating
Metal complexes are useful catalysts for epoxidations involving
Organic peroxides are used for the production of propylene oxide from propylene. Catalysts are required as well. Both
Olefin peroxidation using peroxycarboxylic acids
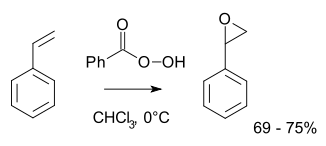
More typically for laboratory operations, the
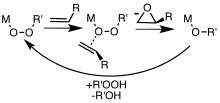
The reaction proceeds via what is commonly known as the "Butterfly Mechanism".[12] The peroxide is viewed as an electrophile, and the alkene a nucleophile. The reaction is considered to be concerted. The butterfly mechanism allows ideal positioning of the O−O sigma star orbital for C−C π electrons to attack.[13] Because two bonds are broken and formed to the epoxide oxygen, this is formally an example of a coarctate transition state.
Hydroperoxides are also employed in catalytic
Homogeneously catalysed asymmetric epoxidations
Chiral epoxides can often be derived enantioselectively from prochiral alkenes. Many metal complexes give active catalysts, but the most important involve titanium, vanadium, and molybdenum.[14][15]
The Sharpless epoxidation reaction is one of the premier enantioselective chemical reactions. It is used to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols.[16][17]
Dehydrohalogenation
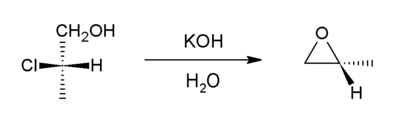
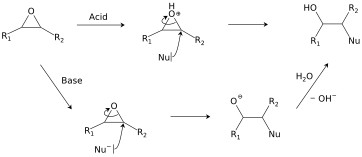
Halohydrins react with base to give epoxides.[19] The reaction is spontaneous because the energetic cost of introducing the ring strain (13 kcal/mol) is offset by the larger bond enthalpy of the newly introduced C-O bond (when compared to that of the cleaved C-halogen bond).
Formation of epoxides from secondary halohydrins is predicted to occur faster than from primary halohydrins due to increased entropic effects in the secondary halohydrin, and tertiary halohydrins react (if at all) extremely slowly due to steric crowding. [20]
Starting with propylene chlorohydrin, most of the world's supply of propylene oxide arises via this route.[8]
An intramolecular epoxide formation reaction is one of the key steps in the Darzens reaction.
In the
Nucleophilic epoxidation
Electron-deficient olefins, such as
Biosynthesis
Epoxides are uncommon in nature. They arise usually via oxygenation of alkenes by the action of cytochrome P450.[21] (but see also the short-lived epoxyeicosatrienoic acids which act as signalling molecules.[22] and similar epoxydocosapentaenoic acids, and epoxyeicosatetraenoic acids.)
Reactions
Ring-opening reactions dominate the reactivity of epoxides.
Hydrolysis and addition of nucleophiles
Epoxides react with a broad range of nucleophiles, for example, alcohols, water, amines, thiols, and even halides. With two often nearly equivalent sites of attack, epoxides are examples "ambident substrates."[23] The regioselectivity of ring-opening in asymmetric epoxides generally follows the SN2 pattern of attack at the least-substituted carbon,[24] but can be affected by carbocation stability under acidic conditions.[25] This class of reactions is the basis of epoxy glues and the production of glycols.[18]
Polymerization and oligomerization
Polymerization of epoxides gives
- ROH + n C2H4O → R(OC2H4)nOH
With anhydrides, epoxides give polyesters.[27]
Deoxygenation
Epoxides can be deoxygenated using
Other reactions
- Reduction of an epoxide with lithium aluminium hydride or aluminium hydride produces the corresponding alcohol.[31]This reduction process results from the nucleophilic addition of hydride (H−).
- Reductive cleavage of epoxides gives β-lithioalkoxides.[32]
- Epoxides undergo ring expansion reactions, illustrated by the insertion of carbon dioxide to give cyclic carbonates.
- When treated with thiourea, epoxides convert to the episulfide, which are called thiiranes.
- An epoxide adjacent to an alcohol can undergo the Payne rearrangement in base.
Uses
-
Bisphenol A diglycidyl ether is a component in common household "epoxy".
-
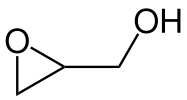
The chemical structure of the epoxide glycidol, a common chemical intermediate.
-
Epothilones are naturally occurring epoxides.
-
3,4-Epoxycyclohexylmethyl-3’,4’-epoxycyclohexane carboxylate, precursor to coatings.[33]
-
Epoxidized linolein, a major component of epoxidized soybean oil (ESBO), a commercially important plasticizer.
-
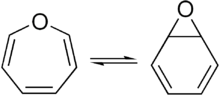
Benzene oxideexists in equilibrium with the oxepin isomer.
Ethylene oxide is widely used to generate detergents and surfactants by ethoxylation. Its hydrolysis affords ethylene glycol. It is also used for sterilisation of medical instruments and materials.
The reaction of epoxides with amines is the basis for the formation of epoxy glues and structural materials. A typical amine-hardener is triethylenetetramine (TETA).
Safety
Epoxides are
See also
Further reading
- Massingill, J. L.; Bauer, R. S. (2000-01-01). "Epoxy Resins". In Craver, Clara D.; Carraher, Charles E. (eds.). Applied Polymer Science: 21st Century. Oxford: Pergamon. pp. 393–424. ISBN 978-0-08-043417-9. Retrieved 2023-12-20.
References
- ISBN 978-3527306732.
- ISBN 978-3527306732.
- .
- .
- S2CID 94240406.
- .
- .
- ^
- ISBN 0-471-85472-7.
- .
- ^ Harold Hibbert and Pauline Burt (1941). "Styrene Oxide". Organic Syntheses; Collected Volumes, vol. 1, p. 494.
- ^ Paul D. Bartlett (1950). "Recent work on the mechanisms of peroxide reactions". Record of Chemical Progress. 11: 47–51.
- ^ John O. Edwards (1962). Peroxide Reaction Mechanisms. Interscience, New York. pp. 67–106.
- .
- .
- .
- Org. Synth., Coll. Vol. 7, p. 461 (1990); Vol. 63, p. 66 (1985). (Article Archived 2013-09-27 at the Wayback Machine)
- ^ ISBN 978-3527306732.
- ^ Koppenhoefer, B.; Schurig, V. (1993). "(R)-Alkyloxiranes of High Enantiomeric Purity from (S)-2-Chloroalkanoic Acids via (S)-2-Chloro-1-Alkanols: (R)-Methyloxirane". Organic Syntheses; Collected Volumes, vol. 8, p. 434.
- .
- PMID 22017381.
- ISBN 978-1-4160-2328-9.
- ISBN 978-0-471-72091-1
- ^ Warren, Stuart; Wyatt, Paul (2008). Organic Synthesis: the disconnection approach (2nd ed.). Wiley. p. 39.
- ^ Rzepa, Henry (28 April 2013). "How to predict the regioselectivity of epoxide ring opening". Chemistry with a twist.
- ISBN 978-3527306732.
- PMID 27936619.
- PMID 26065934.
- .
- .
- .
- .
- .
- S2CID 847639.






![3,4-Epoxycyclohexylmethyl-3’,4’-epoxycyclohexane carboxylate, precursor to coatings.[33]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0e/Diepoxyester.svg/175px-Diepoxyester.svg.png)