Ether
In
Structure and bonding
Ethers feature bent C−O−C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm.[3] The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is similar. In the language of valence bond theory, the hybridization at oxygen is sp3.
Oxygen is more
Ethers can be symmetrical of the type ROR or unsymmetrical of the type ROR'. Examples of the former are
Vinyl- and acetylenic ethers
Vinyl- and acetylenic ethers are far less common than alkyl or aryl ethers. Vinylethers, often called enol ethers, are important intermediates in organic synthesis. Acetylenic ethers are especially rare. Di-tert-butoxyacetylene is the most common example of this rare class of compounds.
Nomenclature
In the
Trivial name
IUPAC rules are often not followed for simple ethers. The trivial names for simple ethers (i.e., those with none or few other functional groups) are a composite of the two substituents followed by "ether". For example, ethyl methyl ether (CH3OC2H5), diphenylether (C6H5OC6H5). As for other organic compounds, very common ethers acquired names before rules for nomenclature were formalized. Diethyl ether is simply called ether, but was once called sweet oil of vitriol. Methyl phenyl ether is
(α-alkoxy ethers R–CH(–OR)–O–R) are another class of ethers with characteristic properties.Polyethers
Polyethers are generally
Crown ethers are cyclic polyethers. Some toxins produced by dinoflagellates such as brevetoxin and ciguatoxin are extremely large and are known as cyclic or ladder polyethers.
| Name of the polymers with low to medium molar mass | Name of the polymers with high molar mass | Preparation | Repeating unit | Examples of trade names |
|---|---|---|---|---|
| Paraformaldehyde | Polyoxymethylene (POM) or polyacetal or polyformaldehyde |
Step-growth polymerisation of formaldehyde |
–CH2O– | Delrin from DuPont |
| Polyethylene glycol (PEG) | Polyethylene oxide (PEO) or polyoxyethylene (POE) | Ring-opening polymerization of ethylene oxide | –CH2CH2O– | Carbowax from Dow |
| Polypropylene glycol (PPG) | Polypropylene oxide (PPOX) or polyoxypropylene (POP) | anionic ring-opening polymerization of propylene oxide | –CH2CH(CH3)O– | Arcol from Covestro |
| Polytetramethylene glycol (PTMG) or Polytetramethylene ether glycol (PTMEG) | Polytetrahydrofuran (PTHF) | Acid-catalyzed ring-opening polymerization of tetrahydrofuran | −CH2CH2CH2CH2O− | Terathane from Invista and PolyTHF from BASF |
The phenyl ether polymers are a class of aromatic polyethers containing aromatic cycles in their main chain: polyphenyl ether (PPE) and poly(p-phenylene oxide) (PPO).
Related compounds
Many classes of compounds with C–O–C linkages are not considered ethers:
There are compounds which, instead of
Physical properties
Ethers have boiling points similar to those of the analogous alkanes. Simple ethers are generally colorless.
| Selected data about some alkyl ethers | |||||
|---|---|---|---|---|---|
| Ether | Structure | m.p. (°C) | b.p. (°C) | Solubility in 1 liter of H2O | Dipole moment (D) |
| Dimethyl ether | CH3–O–CH3 | −138.5 | −23.0 | 70 g | 1.30 |
| Diethyl ether | CH3CH2–O–CH2CH3 | −116.3 | 34.4 | 69 g | 1.14 |
| Tetrahydrofuran | O(CH2)4 | −108.4 | 66.0 | Miscible | 1.74 |
| Dioxane | O(C2H4)2O | 11.8 | 101.3 | Miscible | 0.45 |
Reactions

The C-O bonds that comprise simple ethers are strong. They are unreactive toward all but the strongest bases. Although generally of low chemical
Specialized ethers such as
Cleavage
Although ethers resist hydrolysis, they are cleaved by hydrobromic acid and hydroiodic acid. Hydrogen chloride cleaves ethers only slowly. Methyl ethers typically afford methyl halides:
- ROCH3 + HBr → CH3Br + ROH
These reactions proceed via
Some ethers undergo rapid cleavage with boron tribromide (even aluminium chloride is used in some cases) to give the alkyl bromide.[5] Depending on the substituents, some ethers can be cleaved with a variety of reagents, e.g. strong base.
Despite these difficulties the chemical paper pulping processes are based on cleavage of ether bonds in the lignin.
Peroxide formation
When stored in the presence of air or oxygen, ethers tend to form
Lewis bases

Ethers serve as
Alpha-halogenation
This reactivity is similar to the tendency of ethers with alpha hydrogen atoms to form peroxides. Reaction with chlorine produces alpha-chloroethers.
Synthesis
Dehydration of alcohols
The dehydration of alcohols affords ethers:[7]
- 2 R–OH → R–O–R + H2O at high temperature

This direct nucleophilic substitution reaction requires elevated temperatures (about 125 °C). The reaction is catalyzed by acids, usually sulfuric acid. The method is effective for generating symmetrical ethers, but not unsymmetrical ethers, since either OH can be protonated, which would give a mixture of products. Diethyl ether is produced from ethanol by this method. Cyclic ethers are readily generated by this approach. Elimination reactions compete with dehydration of the alcohol:
- R–CH2–CH2(OH) → R–CH=CH2 + H2O
The dehydration route often requires conditions incompatible with delicate molecules. Several milder methods exist to produce ethers.
Electrophilic addition of alcohols to alkenes
Alcohols add to electrophilically activated alkenes. The method is atom-economical:
- R2C=CR2 + R–OH → R2CH–C(–O–R)–R2
Epoxides
Epoxides are typically prepared by oxidation of alkenes. The most important epoxide in terms of industrial scale is ethylene oxide, which is produced by oxidation of ethylene with oxygen. Other epoxides are produced by one of two routes:
- By the oxidation of alkenes with a peroxyacid such as m-CPBA.
- By the base intramolecular nucleophilic substitution of a halohydrin.
Many ethers,
Williamson and Ullmann ether syntheses
- R–ONa + R′–X → R–O–R′ + NaX
This reaction, the Williamson ether synthesis, involves treatment of a parent alcohol with a strong base to form the alkoxide, followed by addition of an appropriate aliphatic compound bearing a suitable leaving group (R–X). Although popular in textbooks, the method is usually impractical on scale because it cogenerates significant waste.
Suitable leaving groups (X) include iodide, bromide, or sulfonates. This method usually does not work well for aryl halides (e.g. bromobenzene, see Ullmann condensation below). Likewise, this method only gives the best yields for primary halides. Secondary and tertiary halides are prone to undergo E2 elimination on exposure to the basic alkoxide anion used in the reaction due to steric hindrance from the large alkyl groups.
In a related reaction, alkyl halides undergo nucleophilic displacement by
- C6H5OH + OH− → C6H5–O− + H2O
- C6H5–O− + R–X → C6H5OR
The Ullmann condensation is similar to the Williamson method except that the substrate is an aryl halide. Such reactions generally require a catalyst, such as copper.[8]
Important ethers

|
Ethylene oxide | A cyclic ether. Also the simplest epoxide. |
| Dimethyl ether | A colourless gas that is used as an aerosol spray propellant. A potential renewable alternative fuel for diesel engines with a cetane rating as high as 56–57.
| |
| Diethyl ether | A colourless liquid with sweet odour. A common low boiling perfumery .
| |
| Dimethoxyethane (DME) | A water miscible solvent often found in lithium batteries (b.p. 85 °C): | |

|
Dioxane
|
A cyclic ether and high-boiling solvent (b.p. 101.1 °C). |

|
Tetrahydrofuran (THF) | A cyclic ether, one of the most polar simple ethers that is used as a solvent. |

|
Anisole (methoxybenzene) | An aryl ether and a major constituent of the essential oil of anise seed. |
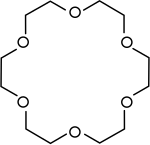
|
Crown ethers | Cyclic polyethers that are used as phase transfer catalysts .
|

|
Polyethylene glycol (PEG) | A linear polyether, e.g. used in pharmaceuticals .
|
| Polypropylene glycol | A linear polyether, e.g. used in polyurethanes .
| |
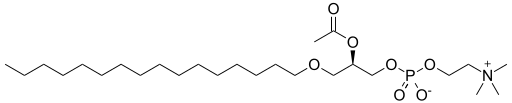
|
Platelet-activating factor | An ether lipid, an example with an ether on sn-1, an ester on sn-2, and an inorganic ether on sn-3 of the glyceryl scaffold. |
See also
- Ester
- Ether lipid
- Ether addiction
- Ether (song)
- History of general anesthesia
- Inhalant
- Chemical paper pulping processes: Kraft process (and Soda pulping), Organosolv pulping process and the Sulfite process
References
- ISBN 978-0-470-77107-5.
- PMID 15303175.
- ^
- ^ J. F. W. McOmie and D. E. West (1973). "3,3′-Dihydroxylbiphenyl". Organic Syntheses; Collected Volumes, vol. 5, p. 412.
- .
- ISBN 978-0-19-850346-0.
- .
