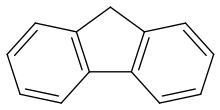
Fluorene
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Fluorene[2] | |
| Systematic IUPAC name
Tricyclo[7.4.0.02,7]trideca-2,4,6,9,11,13-hexaene | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.001.541 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
RTECS number
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H10 | |
| Molar mass | 166.223 g·mol−1 |
| Density | 1.202 g/mL |
| Melting point | 116 to 117 °C (241 to 243 °F; 389 to 390 K) |
| Boiling point | 295 °C (563 °F; 568 K) |
| 1.992 mg/L | |
| Solubility | organic solvents |
| log P | 4.18 |
| Acidity (pKa) | 22.6 |
| -110.5·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 152 °C (306 °F; 425 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
16000 mg/kg (oral, rat) |
| Safety data sheet (SDS) | Sigma-Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluorene /ˈflʊəriːn/, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar.[3] It is insoluble in water and soluble in many organic solvents. Although sometimes classified as a polycyclic aromatic hydrocarbon, the five-membered ring has no aromatic properties.[citation needed] Fluorene is mildly acidic.
Synthesis, structure, and reactivity
Although fluorene is obtained from coal tar, it can also be prepared by dehydrogenation of diphenylmethane.[3] Alternatively, it can be prepared by the reduction of fluorenone with zinc[4] or hypophosphorous acid–iodine.[5] The fluorene molecule is nearly planar,[6] although each of the two benzene rings is coplanar with the central carbon 9.[7]
Fluorene can be found after the
Acidity
The C9-H sites of the fluorene ring are weakly acidic (
Both protons can be removed from C9. For example, 9,9-fluorenyldipotassium can be obtained by treating fluorene with
Ligand properties
Fluorene and its derivatives can be deprotonated to give
Uses
Fluorene is a precursor to other fluorene compounds; the parent species has few applications. Fluorene-9-carboxylic acid is a precursor to pharmaceuticals. Oxidation of fluorene gives
Fluorene dyes
Fluorene dyes are well developed. Most are prepared by condensation of the active methylene group with carbonyls. 2-Aminofluorene, 3,6-bis-(dimethylamino)fluorene, and 2,7-diiodofluorene are precursors to dyes.[12]
See also
References
- ^ Merck Index, 11th Edition, 4081
- ISBN 978-0-85404-182-4.
- ^ ISBN 978-3527306732.
- ISSN 0040-4039.
- doi:10.1038/173635a0
- .
- ISSN 0888-5885.
- .
- doi:10.1139/v60-100..
- PMID 22148816.
- S2CID 95312830.
External links
- Fluorene in the National Institute of Standards and Technology database.

