Fullerene


| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
A fullerene is an
Fullerenes with a closed mesh topology are informally denoted by their empirical formula Cn, often written Cn, where n is the number of carbon atoms. However, for some values of n there may be more than one isomer.
The family is named after
Fullerenes had been predicted for some time, but only after their accidental synthesis in 1985 were they detected in nature[3][4] and outer space.[5][6] The discovery of fullerenes greatly expanded the number of known allotropes of carbon, which had previously been limited to graphite, diamond, and amorphous carbon such as soot and charcoal. They have been the subject of intense research, both for their chemistry and for their technological applications, especially in materials science, electronics, and nanotechnology.[7]
Definition
IUPAC defines fullerenes as "polyhedral closed cages made up entirely of n three-coordinate carbon atoms and having 12 pentagonal and (n/2-10) hexagonal faces, where n ≥ 20."[8]
History
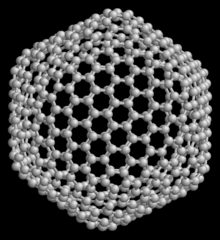
540, another member of the family of fullerenes
Predictions and limited observations
The icosahedral C
60H
60 cage was mentioned in 1965 as a possible topological structure.[9] Eiji Osawa predicted the existence of C
60 in 1970.[10][11] He noticed that the structure of a corannulene molecule was a subset of the shape of a football, and hypothesised that a full ball shape could also exist. Japanese scientific journals reported his idea, but neither it nor any translations of it reached Europe or the Americas.
Also in 1970, R.W.Henson (then of the UK Atomic Energy Research Establishment) proposed the C
60 structure and made a model of it. Unfortunately, the evidence for that new form of carbon was very weak at the time, so the proposal was met with skepticism, and was never published. It was acknowledged only in 1999.[12][13]
In 1973, independently from Henson, D. A. Bochvar and E. G. Galpern made a quantum-chemical analysis of the stability of C
60 and calculated its electronic structure. The paper was published in 1973,[14] but the scientific community did not give much importance to this theoretical prediction.
Around 1980, Sumio Iijima identified the molecule of C
60 from an electron microscope image of carbon black, where it formed the core of a particle with the structure of a "bucky onion".[15]
Also in the 1980s at MIT, Mildred Dresselhaus and Morinobu Endo, collaborating with T. Venkatesan, directed studies blasting graphite with lasers, producing carbon clusters of atoms, which would be later identified as "fullerenes."[16]
Discovery of C
60
In 1985,
60 and C
70. The team identified their structure as the now familiar "buckyballs".[17]
The name "buckminsterfullerene" was eventually chosen for C
60 by the discoverers as an homage to
Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry[19] for their roles in the discovery of this class of molecules.
Further developments
Kroto and the Rice team already discovered other fullerenes besides C60,
After their discovery, minute quantities of fullerenes were found to be produced in sooty flames,[22] and by lightning discharges in the atmosphere.[4] In 1992, fullerenes were found in a family of mineraloids known as shungites in Karelia, Russia.[3]
The production techniques were improved by many scientists, including
In 2010, the spectral signatures of C60 and C70 were observed by NASA's Spitzer infrared telescope in a cloud of cosmic dust surrounding a star 6500 light years away.[5] Kroto commented: "This most exciting breakthrough provides convincing evidence that the buckyball has, as I long suspected, existed since time immemorial in the dark recesses of our galaxy."[6] According to astronomer Letizia Stanghellini, "It’s possible that buckyballs from outer space provided seeds for life on Earth."[24] In 2019, ionized C60 molecules were detected with the Hubble Space Telescope in the space between those stars.[25][26]
Types
There are two major families of fullerenes, with fairly distinct properties and applications: the closed buckyballs and the open-ended cylindrical carbon nanotubes.[27] However, hybrid structures exist between those two classes, such as carbon nanobuds — nanotubes capped by hemispherical meshes or larger "buckybuds".
Buckyballs
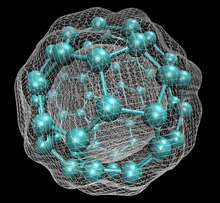
60 with isosurface of ground state electron density as calculated with DFT
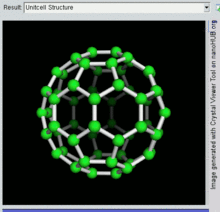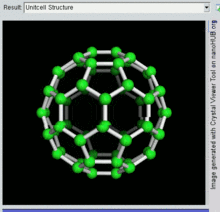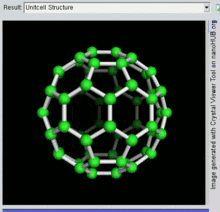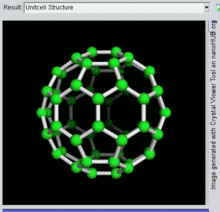
60, one kind of fullerene
Buckminsterfullerene
Buckminsterfullerene is the smallest fullerene molecule containing pentagonal and hexagonal rings in which no two pentagons share an edge (which can be destabilizing, as in pentalene). It is also most common in terms of natural occurrence, as it can often be found in soot.
The empirical formula of buckminsterfullerene is C
60 and its structure is a
The
The buckminsterfullerene molecule has two bond lengths. The 6:6 ring bonds (between two hexagons) can be considered "double bonds" and are shorter (1.401 Å) than the 6:5 bonds (1.458 Å, between a hexagon and a pentagon). The weighted average bond length is 1.44 Å.[29]
Other fullerenes
Another fairly common fullerene has empirical formula C
70,[30] but fullerenes with 72, 76, 84 and even up to 100 carbon atoms are commonly obtained.
The smallest possible fullerene is the dodecahedral C
20. There are no fullerenes with 22 vertices.[31] The number of different fullerenes C2n grows with increasing n = 12, 13, 14, ..., roughly in proportion to n9 (sequence A007894 in the OEIS). For instance, there are 1812 non-isomorphic fullerenes C
60. Note that only one form of C
60, buckminsterfullerene, has no pair of adjacent pentagons (the smallest such fullerene). To further illustrate the growth, there are 214,127,713 non-isomorphic fullerenes C
200, 15,655,672 of which have no adjacent pentagons. Optimized structures of many fullerene isomers are published and listed on the web.[32]
Heterofullerenes have heteroatoms substituting carbons in cage or tube-shaped structures. They were discovered in 1993[33] and greatly expand the overall fullerene class of compounds and can have dangling bonds on their surfaces. Notable examples include boron, nitrogen (azafullerene), oxygen, and phosphorus derivatives.
Carbon nanotubes
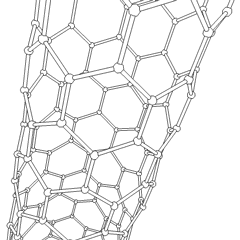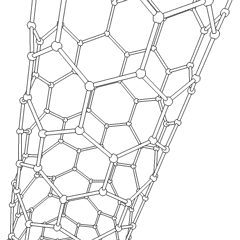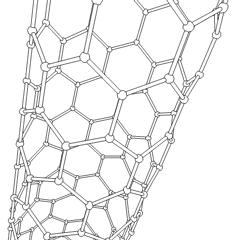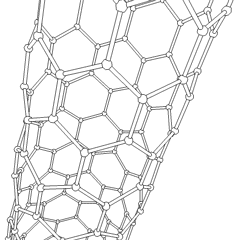
Derivatives
Buckyballs and carbon nanotubes have been used as building blocks for a great variety of derivatives and larger structures, such as[27]
- Nested buckyballs ("carbon nano-onions" or "buckyonions")[35] proposed for lubricants;[36]
- Nested carbon nanotubes ("carbon megatubes")[37]
- Linked "ball-and-chain" dimers (two buckyballs linked by a carbon chain)[38]
- Rings of buckyballs linked together.[39]
Heterofullerenes and non-carbon fullerenes
After the discovery of C60, many fullerenes have been synthesized (or studied theoretically by
Boron
A type of buckyball which uses boron atoms, instead of the usual carbon, was predicted and described in 2007. The B
80 structure, with each atom forming 5 or 6 bonds, was predicted to be more stable than the C
60 buckyball.[40] However, subsequent analysis found that the predicted Ih symmetric structure was vibrationally unstable and the resulting cage would undergo a spontaneous symmetry break, yielding a puckered cage with rare Th symmetry (symmetry of a volleyball).[41] The number of six-member rings in this molecule is 20 and number of five-member rings is 12. There is an additional atom in the center of each six-member ring, bonded to each atom surrounding it. By employing a systematic global search algorithm, it was later found that the previously proposed b
80 fullerene is not a global maximum for 80-atom boron clusters and hence can not be found in nature; the most stable configurations have complex.[42] The same paper concluded that boron's energy landscape, unlike others, has many disordered low-energy structures, hence pure boron fullerenes are unlikely to exist in nature.[42]
However, an irregular B
40 complex dubbed borospherene was prepared in 2014. This complex has two hexagonal faces and four heptagonal faces with in D2d symmetry interleaved with a network of 48 triangles.[43]
Other elements
Inorganic (carbon-free) fullerene-type structures have been built with the
Icosahedral or distorted-icosahedral fullerene-like complexes have also been prepared for germanium, tin, and lead; some of these complexes are spacious enough to hold most transition metal atoms.[45][46]
Main fullerenes
Below is a table of main closed carbon fullerenes synthesized and characterized so far, with their
| Formula | Num. Isom.[1] |
Mol. Symm. |
Cryst. Symm. |
Space group | No | Pearson symbol |
a (nm) | b (nm) | c (nm) | β° | Z | ρ (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C 20 |
1 | Ih | ||||||||||
| C 60 |
1 | Ih | ||||||||||
| C 70 |
1 | D5h | ||||||||||
| C 72 |
1 | D6h | ||||||||||
| C 74 |
1 | D3h | ||||||||||
| C 76 |
2 | D2* | Monoclinic | P21 | 4 | mP2 | 1.102 | 1.108 | 1.768 | 108.10 | 2 | 1.48 |
| Cubic | Fm3m | 225 | cF4 | 1.5475 | 1.5475 | 1.5475 | 90 | 4 | 1.64 | |||
| C 78 |
5 | D2v | ||||||||||
| C 80 |
7 | |||||||||||
| C 82 |
9 | C 2, C2v, C3v |
Monoclinic | P21 | 4 | mP2 | 1.141 | 1.1355 | 1.8355 | 108.07 | 2 | |
| C 84 |
24 | D2*, D2d | Cubic | Fm3m | 1.5817[50] | 1.5817 | 1.5817 | 90 | ||||
| C 86 |
19 | |||||||||||
| C 88 |
35 | |||||||||||
| C 90 |
46 | |||||||||||
| C 3996 |
In the table, "Num.Isom." is the number of possible isomers within the "isolated pentagon rule", which states that two pentagons in a fullerene should not share edges.[51][52] "Mol.Symm." is the symmetry of the molecule,[52][53] whereas "Cryst.Symm." is that of the crystalline framework in the solid state. Both are specified for the most experimentally abundant form(s). The asterisk * marks symmetries with more than one chiral form.
When C
76 or C
82 crystals are grown from toluene solution they have a monoclinic symmetry. The crystal structure contains toluene molecules packed between the spheres of the fullerene. However, evaporation of the solvent from C
76 transforms it into a face-centered cubic form. fullerenes.
Properties
Topology
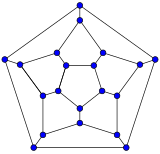
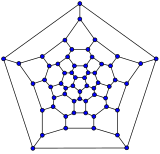
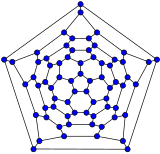
Schlegel diagrams are often used to clarify the 3D structure of closed-shell fullerenes, as 2D projections are often not ideal in this sense.[55]
In mathematical terms, the
- Schlegel diagrams of some fullerenes
-
C20
(dodecahedron) -
C26
-
C60
(truncated icosahedron) -
C70
The Schlegel diagram of a closed fullerene is a graph that is planar and 3-regular (or "cubic"; meaning that all vertices have degree 3).
A closed fullerene with sphere-like shell must have at least some cycles that are pentagons or heptagons. More precisely, if all the faces have 5 or 6 sides, it follows from Euler's polyhedron formula, V−E+F=2 (where V, E, F are the numbers of vertices, edges, and faces), that V must be even, and that there must be exactly 12 pentagons and V/2−10 hexagons. Similar constraints exist if the fullerene has heptagonal (seven-atom) cycles.[56]
Bonding
Since each carbon atom is connected to only three neighbors, instead of the usual four, it is customary to describe those bonds as being a mixture of
Encapsulation
Additional atoms, ions, clusters, or small molecules can be trapped inside fullerenes to form
84, which violates the isolated pentagon rule.[59] Evidence for a meteor impact at the end of the Permian period was found by analyzing noble gases preserved by being trapped in fullerenes.[60]
Research
In the field of
There are many calculations that have been done using
Fullerene is an unusual reactant in many organic reactions such as the Bingel reaction discovered in 1993.
Aromaticity
Researchers have been able to increase the reactivity of fullerenes by attaching active groups to their surfaces. Buckminsterfullerene does not exhibit "
A spherical fullerene of n carbon atoms has n
As a result, C
60 in water tends to pick up two more electrons and become an
Reactions
Polymerization
Under high pressure and temperature, buckyballs collapse to form various one-, two-, or three-dimensional carbon frameworks. Single-strand polymers are formed using the Atom Transfer Radical Addition Polymerization (ATRAP) route.[62]
"Ultrahard fullerite" is a coined term frequently used to describe material produced by high-pressure high-temperature (HPHT) processing of fullerite. Such treatment converts fullerite into a nanocrystalline form of diamond which has been reported to exhibit remarkable mechanical properties.[63]

Chemistry
Fullerenes are stable, but not totally unreactive. The sp2-hybridized carbon atoms, which are at their energy minimum in planar graphite, must be bent to form the closed sphere or tube, which produces angle strain. The characteristic reaction of fullerenes is electrophilic addition at 6,6-double bonds, which reduces angle strain by changing sp2-hybridized carbons into sp3-hybridized ones. The change in hybridized orbitals causes the bond angles to decrease from about 120° in the sp2 orbitals to about 109.5° in the sp3 orbitals. This decrease in bond angles allows for the bonds to bend less when closing the sphere or tube, and thus, the molecule becomes more stable.
Solubility

60 in solution

60 in extra virgin olive oil, showing the characteristic purple color of pristine C
60 solutions
Fullerenes are soluble in many organic
Solutions of pure buckminsterfullerene have a deep purple color. Solutions of C
70 are a reddish brown. The
76 to C
84 have a variety of colors.
Millimeter-sized crystals of C
60 and C
70, both pure and solvated, can be grown from benzene solution. Crystallization of C
60 from benzene solution below 30 °C (when solubility is maximum) yields a
Quantum mechanics
In 1999, researchers from the
Superconductivity
Fullerenes are normally electrical insulators, but when crystallized with alkali metals, the resultant compound can be conducting or even superconducting.[72]
Chirality
Some fullerenes (e.g. C
76, C
78, C
80, and C
84) are inherently chiral because they are D2-symmetric, and have been successfully resolved. Research efforts are ongoing to develop specific sensors for their enantiomers.
Stability
Two theories have been proposed to describe the molecular mechanisms that make fullerenes. The older, “bottom-up” theory proposes that they are built atom-by-atom. The alternative “top-down” approach claims that fullerenes form when much larger structures break into constituent parts.[73]
In 2013 researchers discovered that asymmetrical fullerenes formed from larger structures settle into stable fullerenes. The synthesized substance was a particular metallofullerene consisting of 84 carbon atoms with two additional carbon atoms and two yttrium atoms inside the cage. The process produced approximately 100 micrograms.[73]
However, they found that the asymmetrical molecule could theoretically collapse to form nearly every known fullerene and metallofullerene. Minor perturbations involving the breaking of a few molecular bonds cause the cage to become highly symmetrical and stable. This insight supports the theory that fullerenes can be formed from graphene when the appropriate molecular bonds are severed.[73][74]
Systematic naming
According to the
60-Ih)[5,6]fullerene. The name of the point group should be retained in any derivative of said fullerene, even if that symmetry is lost by the derivation.
To indicate the position of substituted or attached elements, the fullerene atoms are usually numbered in a spiral path, usually starting with the ring on one of the main axes. If the structure of the fullerene does not allow such numbering, another starting atom was chosen to still achieve a spiral path sequence.
The latter is the case for C70, which is (C
70-D5h(6))[5,6]fullerene in IUPAC notation. The symmetry D5h(6) means that this is the isomer where the C5 axis goes through a pentagon surrounded by hexagons rather than pentagons.[55]
-
(C
60-Ih)[5,6]fullerene
Carbon numbering. -
(C
70-D5h(6))[5,6]fullerene
Carbon numbering. -
(C
70-D5h(6))[5,6]fullerene
Non-equivalent bonds shown by different colours. -
3'H-Cyclopropa[1,2](C
70-D5h(6))[5,6]fullerene. -
3'H-Cyclopropa[2,12](C
70-D5h(6))[5,6]fullerene. -
C
71-PCBM, [1,2]-isomer.
IUPAC name is methyl 4-(3’-phenyl-3’H-cyclopropa[1,2](C
70-D5h(6))[5,6]fullerene-3’-yl)butyrate.
In IUPAC's nomenclature, fully saturated analogues of fullerenes are called fulleranes. If the mesh has other element(s) substituted for one or more carbons, the compound is named a heterofullerene. If a double bond is replaced by a methylene bridge −CH2−, the resulting structure is a homofullerene. If an atom is fully deleted and missing valences saturated with hydrogen atoms, it is a norfullerene. When bonds are removed (both sigma and pi), the compound becomes secofullerene; if some new bonds are added in an unconventional order, it is a cyclofullerene.[55]
Production
Fullerene production generally starts by producing fullerene-rich soot. The original (and still current) method was to send a large electric current between two nearby
These processes yield a mixture of various fullerenes and other forms of carbon. The fullerenes are then extracted from the soot using appropriate organic solvents and separated by chromatography.[78]: p.369 One can obtain milligram quantities of fullerenes with 80 atoms or more. C76, C78 and C84 are available commercially.
Applications
Biomedical
Functionalized fullerenes have been researched extensively for several potential biomedical applications including high-performance MRI contrast agents, X-ray imaging contrast agents, photodynamic therapy for tumor treatment,[79][80] and drug and gene delivery.[81][82]
Safety and toxicity
In 2013, a comprehensive review on the toxicity of fullerene was published reviewing work beginning in the early 1990s to present and concluded that very little evidence gathered since the discovery of fullerenes indicate that C
60 is toxic.[81] The toxicity of these carbon nanoparticles is not only dose- and time-dependent, but also depends on a number of other factors such as:
- type (e.g.: C
60, C
70, M@C
60, M@C
82) - functional groups used to water-solubilize these nanoparticles (e.g.: OH, COOH)
- method of administration (e.g.: intravenous, intraperitoneal)
It was recommended to assess the pharmacology of every new fullerene- or metallofullerene-based complex individually as a different compound.
Popular culture
Examples of fullerenes appear frequently in popular culture. Fullerenes appeared in fiction well before scientists took serious interest in them. In a humorously speculative 1966 column for New Scientist, David Jones suggested the possibility of making giant hollow carbon molecules by distorting a plane hexagonal net with the addition of impurity atoms.[83]
See also
- Buckypaper
- Carbocatalysis
- Dodecahedrane
- Fullerene ligand
- Goldberg–Coxeter construction
- Lonsdaleite
- Triumphene
- Truncated rhombic triacontahedron
References
- ^ "Definition of BUCKYTUBE". www.merriam-webster.com.
- ^ "fullerite". Archived from the original on 23 October 2015.
- ^ S2CID 4956299.
- ^ a b "The allotropes of carbon". Interactive Nano-Visualization in Science & Engineering Education. Archived from the original on 18 June 2010. Retrieved 29 August 2010.
- ^ S2CID 33588270.
- ^ a b Stars reveal carbon 'spaceballs', BBC, 22 July 2010.
- PMID 25662746.
- S2CID 94299129.
- .
- ^ Osawa, E. (1970). "Superaromaticity". Kagaku. 25: 854–863.
- .
- .
- ^ Henson, R.W. "The History of Carbon 60 or Buckminsterfullerene". Archived from the original on 15 June 2013.
- ^ Bochvar, D.A.; Galpern, E.G. (1973). "О гипотетических системах: карбододекаэдре, s-икосаэдре и карбо-s-икосаэдре" [On hypothetical systems: carbon dodecahedron, S-icosahedron and carbon-S-icosahedron]. Dokl. Akad. Nauk SSSR. 209: 610.
- .
- ^ "Mildred S. Dresselhaus". The Franklin Institute. 5 October 2016. Retrieved 8 October 2022.
- ^ S2CID 4314237.
- ^ Buckminsterfullerene, C
60. Sussex Fullerene Group. chm.bris.ac.uk - ^ "The Nobel Prize in Chemistry 1996". Retrieved 7 February 2014.
- ^ Mraz, S.J. (14 April 2005). "A new buckyball bounces into town". Machine Design. Archived from the original on 13 October 2008.
- S2CID 4302490.
- ISSN 0002-7863.
- S2CID 4359360.
- ^ Atkinson, Nancy (27 October 2010). "Buckyballs Could Be Plentiful in the Universe". Universe Today. Retrieved 28 October 2010.
- ^ Starr, Michelle (29 April 2019). "The Hubble Space Telescope Has Just Found Solid Evidence of Interstellar Buckyballs". ScienceAlert.com. Retrieved 29 April 2019.
- S2CID 121292704.
- ^ ISBN 978-0-13-120198-9.
- PMID 17316055.
- ISSN 0036-8075.
- Imperial College. Retrieved 4 July 2010.
- S2CID 95413107.
- ^ Fowler, P. W. and Manolopoulos, D. E. C
n Fullerenes. nanotube.msu.edu - ^ Harris, D.J. "Discovery of Nitroballs: Research in Fullerene Chemistry" http://www.usc.edu/CSSF/History/1993/CatWin_S05.html Archived 29 November 2015 at the Wayback Machine
- PMID 17699622.
- S2CID 2695746.
- S2CID 4431690.
- PMID 11375691.
- .
- .
- PMID 17501448.
- S2CID 97264790.
- ^ S2CID 16414023.
- PMID 25054944.
- ^ Genuth, Iddo; Yaffe, Tomer (15 February 2006). "Protecting the soldiers of tomorrow". IsraCast. Archived from the original on 26 March 2008.
- PMID 16802791.
- PMID 16928103.
- ISBN 978-1-85617-567-8. Retrieved 26 December 2011.
- PMID 16833800.
- .
- .
- .
- ^ .
- ISBN 978-81-224-1142-3. Retrieved 26 December 2011.
- PMID 9977506.
- ^ S2CID 93423610.
- ^ "Fullerene", Encyclopædia Britannica on-line
- ^ S2CID 224784081.
- S2CID 93478144.
- PMID 16939248.
- S2CID 45230096.
- PMID 15706578.
- .
- .
- .
- S2CID 250785669.
- doi:10.1021/j100115a049. Archived from the original(PDF) on 8 May 2012. Retrieved 24 February 2015.
- .
- .
- .
- .
- S2CID 4424892.
- ISBN 978-0-444-52844-5.
- ^ a b c Support for top-down theory of how 'buckyballs’ form. kurzweilai.net. 24 September 2013
- PMID 24056346.
- ^ Bobrowsky, Maciej (1 October 2019). "Nanostructures and computer simulations in material science" (PDF). Retrieved 3 February 2020.
- ISBN 978-0-7923-7174-8.
- ISSN 1660-6795.
- ISBN 978-0-444-52844-5.
- PMID 22108244.
- PMID 17664135.
- ^ a b G. Lalwani and B. Sitharaman, Multifunctional fullerene and metallofullerene based nanobiomaterials, NanoLIFE 08/2013; 3:1342003. DOI: 10.1142/S1793984413420038 Full Text PDF
- .
- ^ Jones, D. (1966). "Note in Ariadne column". New Scientist. 32: 245.
External links
- Nanocarbon: From Graphene to Buckyballs Interactive 3D models of cyclohexane, benzene, graphene, graphite, chiral & non-chiral nanotubes, and C60 Buckyballs – WeCanFigureThisOut.org.
- Introduction to fullerites
- Giant Fullerenes, a short video looking at Giant Fullerenes


![(C 60-Ih)[5,6]fullerene Carbon numbering.](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c8/Buckminsterfullerene-2D-skeletal_numbered.svg/200px-Buckminsterfullerene-2D-skeletal_numbered.svg.png)
![(C 70-D5h(6))[5,6]fullerene Carbon numbering.](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2c/C70fullerene-2D-skeletal_numbered.svg/187px-C70fullerene-2D-skeletal_numbered.svg.png)
![(C 70-D5h(6))[5,6]fullerene Non-equivalent bonds shown by different colours.](http://upload.wikimedia.org/wikipedia/commons/thumb/9/99/C70fullerene-2D-skeletal_numbered_isobonds.svg/187px-C70fullerene-2D-skeletal_numbered_isobonds.svg.png)
)[5,6]fullerene.](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d7/Cyclopropa12_C70fullerene-2D-skeletal_renumbered.svg/198px-Cyclopropa12_C70fullerene-2D-skeletal_renumbered.svg.png)
)[5,6]fullerene.](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c3/Cyclopropa212_C70fullerene-2D-skeletal_renumbered.svg/198px-Cyclopropa212_C70fullerene-2D-skeletal_renumbered.svg.png)
![C 71-PCBM, [1,2]-isomer. IUPAC name is methyl 4-(3’-phenyl-3’H-cyclopropa[1,2](C 70-D5h(6))[5,6]fullerene-3’-yl)butyrate.](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d3/PC71BM.svg/200px-PC71BM.svg.png)