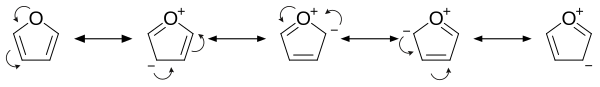Furan
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Furan[1] | |||
| Systematic IUPAC name
1,4-Epoxybuta-1,3-diene 1-Oxacyclopenta-2,4-diene | |||
| Other names
Oxole
Oxa[5]annulene 1,4-Epoxy-1,3-butadiene 5-Oxacyclopenta-1,3-diene 5-Oxacyclo-1,3-pentadiene Furfuran Divinylene oxide | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| 103221 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
ECHA InfoCard
|
100.003.390 | ||
| EC Number |
| ||
| 25716 | |||
| KEGG | |||
PubChem CID
|
|||
RTECS number
|
| ||
| UNII | |||
| UN number | 2389 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4O | |||
| Molar mass | 68.075 g·mol−1 | ||
| Appearance | Colorless, volatile liquid | ||
| Density | 0.936 g/mL | ||
| Melting point | −85.6 °C (−122.1 °F; 187.6 K) | ||
| Boiling point | 31.3 °C (88.3 °F; 304.4 K) | ||
| -43.09·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H224, H302, H315, H332, H341, H350, H373, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P308+P313, P312, P314, P321, P330, P332+P313, P362, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −36 °C (−33 °F; 237 K) | ||
| 390 °C (734 °F; 663 K) | |||
Explosive limits
|
Lower: 2.3% Upper: 14.3% at 20 °C | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
> 2 g/kg (rat) | ||
| Safety data sheet (SDS) | Pennakem | ||
| Related compounds | |||
Related
heterocycles |
Pyrrole Thiophene | ||
Related compounds
|
Tetrahydrofuran (THF) 2,5-Dimethylfuran Benzofuran Dibenzofuran | ||
| Structure | |||
| C2v | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
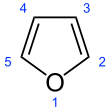
Furan is a
atom. Chemical compounds containing such rings are also referred to as furans.Furan is a colorless,
History
The name "furan" comes from the
Production
Industrially, furan is manufactured by the
In the laboratory, furan can be obtained from furfural by oxidation to 2-furoic acid, followed by decarboxylation.[8] It can also be prepared directly by thermal decomposition of pentose-containing materials, and cellulosic solids, especially pine wood.
Synthesis of furans
The
Many routes exist for the synthesis of substituted furans.[11][12]
- Furan in nature and commerce
-
The drug Zantac, also known as ranitidine.
-
Furfural, derived from sugars, is the major source of furans
-
methanofuran is a cofactor in methanogenesis.
Structure and bonding
Furan has
Examination of the resonance contributors shows the increased electron density of the ring, leading to increased rates of electrophilic substitution.[14]
Reactivity
Because of its partial aromatic character, furan's behavior is intermediate between that of an enol ether and an aromatic ring. It is dissimilar vs ethers such as tetrahydrofuran.
Like enol ethers, 2,5-disubstituted furans are susceptible to hydrolysis to reversibly give 1,4-diketones.
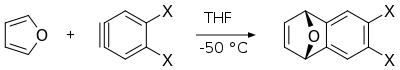
Furan serves as a
Diels-Alder reaction of furan with arynes provides corresponding derivatives of dihydronaphthalenes, which are useful intermediates in synthesis of other polycyclic aromatic compounds.[16]
- It is considerably more reactive than benzene in electrophilic substitution reactions, due to the electron-donating effects of the oxygen heteroatom. It reacts with bromine at 0 °C to give 2-bromofuran.
- Hydrogenation of furans sequentially affords dihydrofurans and tetrahydrofurans.[citation needed]
- In the Achmatowicz reaction, furans are converted to dihydropyran compounds.
- Al2O3.[17]
Safety
Furan is found in heat-treated commercial foods and is produced through
Exposure to furan at doses about 2,000 times the projected level of human exposure from foods increases the risk of hepatocellular tumors in rats and mice and bile duct tumors in rats.[23] Furan is therefore listed as a possible human carcinogen.[23]
See also
- BS 4994 – Furan resin as thermoset FRP for chemical process plant equipments
- Furanocoumarin
- Furanoflavonoid
- Furanose
- Furantetracarboxylic acid
- Simple aromatic rings
- Furan fatty acids
- Tetrahydrofuran
References
- ISBN 978-0-85404-182-4.
- ISBN 0-89925-457-8.
- ^ DHHS (NIOSH) Publication No. 2016–171, p. 2, Accessed Nov 2019
- ^ ISBN 978-3527306732.
- ISBN 0-444-52239-5.
- .
- ^ Rodd, Ernest Harry (1971). Chemistry of Carbon Compounds: A Modern Comprehensive Treatise. Elsevier.
- ^ Wilson, W. C. (1941). "Furan". Organic Syntheses; Collected Volumes, vol. 1, p. 274.
- .
- ^ a b Gilchrist, Thomas L. (1997). Heterocyclic Chemistry (3rd ed.). Liverpool: Longman. p. 209-212.
- doi:10.15227/orgsyn.084.0199.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - .
- ISBN 978-0-471-72091-1
- ISBN 978-0-13-196316-0.
- PMID 16674802.
- PMID 23205621. Archived from the original(PDF) on 2020-02-19.
- ISBN 978-3527306732.
- PMID 23627283. Archived from the original(PDF) on 2017-08-08.
- ^ S2CID 12446132.
- S2CID 29027966.
- Science Daily. April 14, 2011.
- ^ S2CID 19389984.




![Rosefuran, an aroma compound found in rose oil.[10]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b6/Rosefuran-2D-skeletal.png/180px-Rosefuran-2D-skeletal.png)