G0 phase
The G0 phase describes a cellular state outside of the replicative cell cycle. Classically[when?], cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation. Thus it was thought of as a resting phase. G0 is now known to take different forms and occur for multiple reasons. For example, most adult neuronal cells, among the most metabolically active cells in the body, are fully differentiated and reside in a terminal G0 phase. Neurons reside in this state, not because of stochastic or limited nutrient supply, but as a part of their developmental program.
G0 was first suggested as a cell state based on early cell cycle studies. When the first studies defined the four phases of the cell cycle using radioactive labeling techniques, it was discovered that not all cells in a population proliferate at similar rates.[1] A population's "growth fraction" – or the fraction of the population that was growing – was actively proliferating, but other cells existed in a non-proliferative state. Some of these non-proliferating cells could respond to extrinsic stimuli and proliferate by re-entering the cell cycle.[2] Early contrasting views either considered non-proliferating cells to simply be in an extended G1 phase or in a cell cycle phase distinct from G1 – termed G0.[3] Subsequent research pointed to a restriction point (R-point) in G1 where cells can enter G0 before the R-point but are committed to mitosis after the R-point.[4] These early studies provided evidence for the existence of a G0 state to which access is restricted. These cells that do not divide further exit G1 phase to enter an inactive stage called quiescent stage.
Diversity of G0 states
Three G0 states exist and can be categorized as either reversible (quiescent) or irreversible (senescent and differentiated). Each of these three states can be entered from the G1 phase before the cell commits to the next round of the cell cycle. Quiescence refers to a reversible G0 state where subpopulations of cells reside in a 'quiescent' state before entering the cell cycle after activation in response to extrinsic signals. Quiescent cells are often identified by low RNA content, lack of cell proliferation markers, and increased label retention indicating low cell turnover.[5][6] Senescence is distinct from quiescence because senescence is an irreversible state that cells enter in response to DNA damage or degradation that would make a cell's progeny nonviable. Such DNA damage can occur from telomere shortening over many cell divisions as well as reactive oxygen species (ROS) exposure, oncogene activation, and cell-cell fusion. While senescent cells can no longer replicate, they remain able to perform many normal cellular functions.[7][8][9][10] Senescence is often a biochemical alternative to the self-destruction of such a damaged cell by apoptosis. In contrast to cellular senescence, quiescence is not a reactive event but part of the core programming of several different cell types. Finally, differentiated cells are stem cells that have progressed through a differentiation program to reach a mature – terminally differentiated – state. Differentiated cells continue to stay in G0 and perform their main functions indefinitely.
Characteristics of quiescent stem cells
Transcriptomes
The
Epigenetic
Many quiescent stem cells, particularly
Regulation of quiescence
Cell cycle regulators
Functional
Post-transcriptional regulation
Post-transcriptional regulation of gene expression via
Response to stress
Stem cells that have been quiescent for a long time often face various environmental stressors, such as
Examples of reversible G0 phase
Tissue stem cells
Stem cells are cells with the unique ability to produce differentiated daughter cells and to preserve their stem cell identity through self-renewal.[12] In mammals, most adult tissues contain tissue-specific stem cells that reside in the tissue and proliferate to maintain homeostasis for the lifespan of the organism. These cells can undergo immense proliferation in response to tissue damage before differentiating and engaging in regeneration. Some tissue stem cells exist in a reversible, quiescent state indefinitely until being activated by external stimuli. Many different types of tissue stem cells exist, including muscle stem cells (MuSCs), neural stem cells (NSCs), intestinal stem cells (ISCs), and many others.
Stem cell quiescence has been recently suggested to be composed of two distinct functional phases, G0 and an 'alert' phase termed GAlert.[13] Stem cells are believed to actively and reversibly transition between these phases to respond to injury stimuli and seem to gain enhanced tissue regenerative function in GAlert. Thus, transition into GAlert has been proposed as an adaptive response that enables stem cells to rapidly respond to injury or stress by priming them for cell cycle entry. In muscle stem cells,
Mature hepatocytes
While a reversible quiescent state is perhaps most important for tissue stem cells to respond quickly to stimuli and maintain proper homeostasis and regeneration, reversible G0 phases can be found in non-stem cells such as mature hepatocytes.[14] Hepatocytes are typically quiescent in normal livers but undergo limited replication (less than 2 cell divisions) during liver regeneration after partial hepatectomy. However, in certain cases, hepatocytes can experience immense proliferation (more than 70 cell divisions) indicating that their proliferation capacity is not hampered by existing in a reversible quiescent state.[14]
Examples of irreversible G0 phase
Senescent cells
Often associated with aging and age-related diseases in vivo, senescent cells can be found in many renewable tissues, including the
Differentiated muscle
During skeletal
Differentiated bone
Of the four major types of bone cells, osteocytes are the most common and also exist in a terminal G0 phase. Osteocytes arise from osteoblasts that are trapped within a self-secreted matrix. While osteocytes also have reduced synthetic activity, they still serve bone functions besides generating structure. Osteocytes work through various mechanosensory mechanisms to assist in the routine turnover over bony matrix.
Differentiated nerve
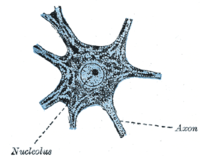
Outside of a few neurogenic niches in the brain, most neurons are fully differentiated and reside in a terminal G0 phase. These fully differentiated neurons form synapses where electrical signals are transmitted by axons to the dendrites of nearby neurons. In this G0 state, neurons continue functioning until senescence or apoptosis. Numerous studies have reported accumulation of DNA damage with age, particularly oxidative damage, in the mammalian brain.[18]
Mechanism of G0 entry
Role of Rim15
Rim15 was first discovered to play a critical role in initiating
In addition to playing a role in meiosis initiation, Rim15 has also been shown to be a critical effector for yeast cell entry into G0 in the presence of stress. Signals from several different nutrient signaling pathways converge on Rim15, which activates the transcription factors, Gis1, Msn2, and Msn4. Gis1 binds to and activates promoters containing post-
Nutrient signaling pathways
Glucose
Yeast grows exponentially through fermentation of glucose. When glucose levels drop, yeast shift from fermentation to cellular respiration, metabolizing the fermentative products from their exponential growth phase. This shift is known as the diauxic shift after which yeast enter G0. When glucose levels in the surroundings are high, the production of cAMP through the RAS-cAMP-PKA pathway (a cAMP-dependent pathway) is elevated, causing protein kinase A (PKA) to inhibit its downstream target Rim15 and allow cell proliferation. When glucose levels drop, cAMP production declines, lifting PKA's inhibition of Rim15 and allowing the yeast cell to enter G0.[19]
Nitrogen
In addition to glucose, the presence of nitrogen is crucial for yeast proliferation. Under low nitrogen conditions, Rim15 is activated to promote cell cycle arrest through inactivation of the protein kinases
Phosphate
Yeast cells respond to low extracellular phosphate levels by activating genes that are involved in the production and upregulation of inorganic phosphate. The PHO pathway is involved in the regulation of phosphate levels. Under normal conditions, the yeast cyclin-dependent kinase complex, Pho80-Pho85, inactivates the Pho4 transcription factor through phosphorylation. However, when phosphate levels drop, Pho81 inhibits Pho80-Pho85, allowing Pho4 to be active. When phosphate is abundant, Pho80-Pho85 also inhibits the nuclear pool of Rim 15 by promoting phosphorylation of its Thr1075 Bmh2 binding site. Thus, Pho80-Pho85 acts in concert with Sch9 and TORC1 to promote cytoplasmic retention of Rim15 under normal conditions.[19]
Mechanism of G0 exit
Cyclin C/Cdk3 and Rb
The transition from G1 to
Rb and G0 exit
Studies suggest that Rb repression of the
References
- ISSN 0020-7616.
- S2CID 86353634.
- PMID 13941891.
- PMID 4524638.
- PMID 11532352.
- PMID 17600112.
- PMID 13905658.
- PMID 23140366.
- PMID 21321098.
- PMID 25080110.
- ^ PMID 23698583.
- PMID 10647940.
- ^ PMID 24870234.
- ^ PMID 15185286.
- PMID 19053174.
- PMID 21719760.
- ^ page 395, Biology, Fifth Edition, Campbell, 1999
- ISBN 978-1604565812
- ^ PMID 16759348.
- ^ PMID 15130482.
- ^ PMID 15084261.
