Glycogen

Glycogen is a multibranched
Glycogen functions as one of three regularly used forms of energy reserves,
In
The amount of glycogen stored in the body mostly depends on oxidative type 1 fibres,[13][14] physical training, basal metabolic rate, and eating habits.[15] Different levels of resting muscle glycogen are reached by changing the number of glycogen particles, rather than increasing the size of existing particles[14] though most glycogen particles at rest are smaller than their theoretical maximum.[16]
Approximately 4 grams of glucose are present in the
Glycogen is an analogue of
Structure
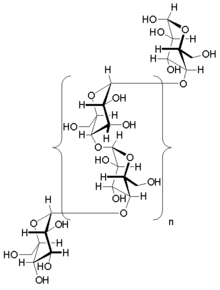
Glycogen is a branched biopolymer consisting of linear chains of glucose residues with an average chain length of approximately 8–12 glucose units and 2,000-60,000 residues per one molecule of glycogen.[20][21]
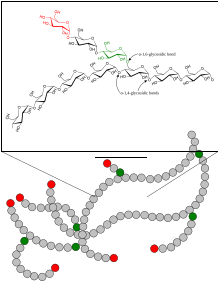
Like amylopectin, glucose units are linked together linearly by α(1→4) glycosidic bonds from one glucose to the next. Branches are linked to the chains from which they are branching off by α(1→6) glycosidic bonds between the first glucose of the new branch and a glucose on the stem chain.[22]
Each glycogen is essentially a ball of glucose trees, with around 12 layers, centered on a glycogenin protein, with three kinds of glucose chains: A, B, and C. There is only one C-chain, attached to the glycogenin. This C-chain is formed by the self-glucosylation of the glycogenin, forming a short primer chain. From the C-chain grows out B-chains, and from B-chains branch out B- and A-chains. The B-chains have on average 2 branch points, while the A-chains are terminal, thus unbranched. On average, each chain has length 12, tightly constrained to be between 11 and 15. All A-chains reach the spherical surface of the glycogen.[23][24]
Glycogen in muscle, liver, and fat cells is stored in a hydrated form, composed of three or four parts of water per part of glycogen associated with 0.45 millimoles (18 mg) of potassium per gram of glycogen.[5]
Glucose is an osmotic molecule, and can have profound effects on osmotic pressure in high concentrations possibly leading to cell damage or death if stored in the cell without being modified.[3] Glycogen is a non-osmotic molecule, so it can be used as a solution to storing glucose in the cell without disrupting osmotic pressure.[3]
Functions
Liver
As a meal containing
After a meal has been digested and glucose levels begin to fall, insulin secretion is reduced, and glycogen synthesis stops. When it is needed for energy, glycogen is broken down and converted again to glucose. Glycogen phosphorylase is the primary enzyme of glycogen breakdown. For the next 8–12 hours, glucose derived from liver glycogen is the primary source of blood glucose used by the rest of the body for fuel.
Glucagon, another hormone produced by the pancreas, in many respects serves as a countersignal to insulin. In response to insulin levels being below normal (when blood levels of glucose begin to fall below the normal range), glucagon is secreted in increasing amounts and stimulates both glycogenolysis (the breakdown of glycogen) and gluconeogenesis (the production of glucose from other sources).
Muscle

Skeletal muscle needs ATP (provides energy) for muscle contraction and relaxation, in what is known as the sliding filament theory. Skeletal muscle relies predominantly on glycogenolysis for the first few minutes as it transitions from rest to activity, as well as throughout high-intensity aerobic activity and all anaerobic activity.[27] During anaerobic activity, such as weightlifting and isometric exercise, the phosphagen system (ATP-PCr) and muscle glycogen are the only substrates used as they do not require oxygen nor blood flow.[27]
Different bioenergetic systems produce ATP at different speeds, with ATP produced from muscle glycogen being much faster than fatty acid oxidation.[28] The level of exercise intensity determines how much of which substrate (fuel) is used for ATP synthesis also. Muscle glycogen can supply a much higher rate of substrate for ATP synthesis than blood glucose. During maximum intensity exercise, muscle glycogen can supply 40 mmol glucose/kg wet weight/minute,[29] whereas blood glucose can supply 4 - 5 mmol.[30][4] Due to its high supply rate and quick ATP synthesis, during high-intensity aerobic activity (such as brisk walking, jogging, or running), the higher the exercise intensity, the more the muscle cell produces ATP from muscle glycogen.[31] This reliance on muscle glycogen is not only to provide the muscle with enough ATP during high-intensity exercise, but also to maintain blood glucose homeostasis (that is, to not become hypoglycaemic by the muscles needing to extract far more glucose from the blood than the liver can provide).[30] A deficit of muscle glycogen leads to muscle fatigue known as "hitting the wall" or "the bonk" (see below under glycogen depletion).
Structure Type
In 1999, Meléndez et al claimed that the structure of glycogen is optimal under a particular metabolic constraint model, where the structure was suggested to be "fractal" in nature.[32] However, research by Besford et al[33] used small angle X-ray scattering experiments accompanied by branching theory models to show that glycogen is a randomly hyperbranched polymer nanoparticle. Glycogen is not fractal in nature. This has been subsequently verified by others who have performed Monte Carlo simulations of glycogen particle growth, and shown that the molecular density reaches a maximum near the centre of the nanoparticle structure, not at the periphery (contradicting a fractal structure that would have greater density at the periphery).[34]
History
Glycogen was discovered by Claude Bernard. His experiments showed that the liver contained a substance that could give rise to reducing sugar by the action of a "ferment" in the liver. By 1857, he described the isolation of a substance he called "la matière glycogène", or "sugar-forming substance". Soon after the discovery of glycogen in the liver, A. Sanson found that muscular tissue also contains glycogen. The empirical formula for glycogen of (C
6H
10O
5)n was established by Kekulé in 1858.[35]
Metabolism
Synthesis
Glycogen synthesis is, unlike its breakdown,
The glycogen branching enzyme catalyzes the transfer of a terminal fragment of six or seven glucose residues from a nonreducing end to the C-6 hydroxyl group of a glucose residue deeper into the interior of the glycogen molecule. The branching enzyme can act upon only a branch having at least 11 residues, and the enzyme may transfer to the same glucose chain or adjacent glucose chains.
Breakdown
Glycogen is cleaved from the nonreducing ends of the chain by the enzyme glycogen phosphorylase to produce monomers of glucose-1-phosphate:
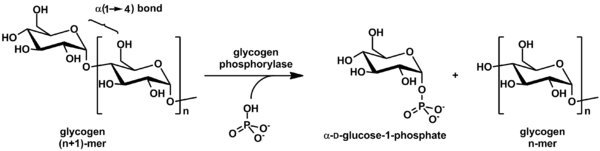
In vivo, phosphorolysis proceeds in the direction of glycogen breakdown because the ratio of phosphate and glucose-1-phosphate is usually greater than 100.
- G6P can continue on the glycolysis pathway and be used as fuel.
- G6P can enter the NADPHand 5 carbon sugars.
- In the liver and kidney, G6P can be dephosphorylated back to glucose by the enzyme glucose 6-phosphatase. This is the final step in the gluconeogenesis pathway.
Clinical relevance
Disorders of glycogen metabolism
The most common disease in which glycogen metabolism becomes abnormal is diabetes, in which, because of abnormal amounts of insulin, liver glycogen can be abnormally accumulated or depleted. Restoration of normal glucose metabolism usually normalizes glycogen metabolism, as well.
In hypoglycemia caused by excessive insulin, liver glycogen levels are high, but the high insulin levels prevent the glycogenolysis necessary to maintain normal blood sugar levels. Glucagon is a common treatment for this type of hypoglycemia.
Various inborn errors of carbohydrate metabolism are caused by deficiencies of enzymes or transport proteins necessary for glycogen synthesis or breakdown. These are collectively referred to as glycogen storage diseases.
Glycogen depletion and endurance exercise
Long-distance athletes, such as
Glycogen depletion can be forestalled in three possible ways:
- First, during exercise, carbohydrates with the highest possible rate of conversion to blood glucose (high glycemic index) are ingested continuously. The best possible outcome of this strategy replaces about 35% of glucose consumed at heart rates above about 80% of maximum.
- Second, through endurance training adaptations and specialized regimens (e.g. fasting, low-intensity endurance training), the body can condition sparing carbohydrate use from all sources.
- Third, by consuming large quantities of carbohydrates after depleting glycogen stores as a result of exercise or diet, the body can increase storage capacity of intramuscular glycogen stores.[13][40][41][42] This process is known as carbohydrate loading. In general, glycemic index of carbohydrate source does not matter since muscular insulin sensitivity is increased as a result of temporary glycogen depletion.[43][44]
When athletes ingest both carbohydrate and
Nanomedicine
Recently, Glycogen Nanoparticles have been investigated as potential drug delivery systems.[47]
See also
References
- ISBN 978-0-7817-4990-9.
- ISBN 9781429254311.
- ^ OCLC 913469736.
- ^ PMID 18840763.
Four grams of glucose circulates in the blood of a person weighing 70 kg. This glucose is critical for normal function in many cell types. In accordance with the importance of these 4 g of glucose, a sophisticated control system is in place to maintain blood glucose constant. Our focus has been on the mechanisms by which the flux of glucose from liver to blood and from blood to skeletal muscle is regulated. ... The brain consumes ~60% of the blood glucose used in the sedentary, fasted person. ... The amount of glucose in the blood is preserved at the expense of glycogen reservoirs (Fig. 2). In postabsorptive humans, there are ~100 g of glycogen in the liver and ~400 g of glycogen in muscle. Carbohydrate oxidation by the working muscle can go up by ~10 fold with exercise, and yet after 1 h, blood glucose is maintained at ~4 g.
- ^ PMID 1615908.
- ISBN 978-5-98657-013-6.
- PMID 5083874.
- .
- PMID 12564847.
- PMID 29444266.
- PMID 27353480.
- ISBN 978-0-13-250882-7.
- ^ S2CID 220653138.
- ^ S2CID 232131416.
- PMID 5584523.
- PMID 12381743.
- PMID 14363101.
- PMID 7151939.
- S2CID 2331300.
- ISSN 0144-8617.
- ISBN 978-1-929007-63-9.
- ISBN 978-1429203142.
- S2CID 34722785.
- PMID 22248338.
- S2CID 249489284. Retrieved 12 May 2023.
- ^ "Glycogen Biosynthesis; Glycogen Breakdown". oregonstate.edu. Retrieved 28 February 2018.
- ^ S2CID 240123241.
- ^ "Hormonal Regulation of Energy Metabolism - Berne and Levy Physiology, 6th ed (2008)". doctorlib.info. Retrieved 21 October 2023.
- PMID 29444266.
- ^ PMID 32785124.
- PMID 11579177.
- hdl:2445/122234.
- hdl:11343/282927.
- .
- PMID 13436813.
- ^ Nelson, D. (2013). Lehninger Principles of Biochemistry (6th ed.). W.H. Freeman and Company. p. 618.
- ^ Stryer, L. (1988). Biochemistry (3rd ed.). Freeman. p. 451.
- ^ "Methods of endurance training, Part 1". 30 October 2009. Archived from the original on 22 July 2018. Retrieved 1 August 2013.
- ^ "Steady state vs. tempo training and fat loss". 2 June 2008. Archived from the original on 5 September 2017. Retrieved 1 August 2013.
- ^ McDonald, Lyle (25 July 2012). "Research review: An in-depth look into carbing up on the cyclical ketogenic diet". Retrieved 19 February 2017.
- ^ McDonald, Lyle (1998). The Ketogenic Diet: A complete guide for the dieter and the practitioner. Lyle McDonald.
- PMID 5123660.
- PMID 3538900.
- ^ McDonald, Lyle (2003). The Ultimate Diet 2.0. Lyle McDonald.
- PMID 18467543.
- ^ S2CID 13748227.
- S2CID 204738366.
External links
- "Glycogen storage disease". McArdle's Diseases.
- Glycogen at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
